Imagine a world where the foods you eat are like musical masterpieces. Every bite is a harmonious blend of flavors and textures. But what if we told you that behind this culinary symphony, there’s a fascinating dance of molecules going on? In the kitchen alchemy called frying, carbon atoms take center stage, transforming into a kaleidoscope of colors, textures, and aromas. So, get ready to explore the sizzling science of frying, where carbon embarks on an epic journey that reveals the secrets of life, death, and the building blocks of our planet.
What Happens to Carbon Atoms When Fried?
We all love that satisfying crunch and delicious smell when we fry food. This transformation is largely due to a chemical reaction with a fancy name: the Maillard reaction. This reaction is like a dance between amino acids (the building blocks of protein) and sugars. As they react in the heat, they create a bunch of new flavor compounds. These compounds are what give fried food that irresistible golden-brown color and savory taste we all crave.
But the oil itself plays a bigger role than you might think. It’s not just there to transfer heat and make things crispy. The type of oil you use can actually change during frying! Some oils, called unsaturated fats, have double bonds between carbon atoms. When exposed to high heat for a while, these double bonds can break and form single bonds instead. This process, known as hydrogenation, transforms unsaturated fats into saturated fats. You’ve probably heard that saturated fats aren’t the healthiest. They’ve been linked to higher levels of “bad” cholesterol, which can increase the risk of heart disease.
And to make things even more interesting, some unsaturated fats can also turn into something called trans fats during frying, which have also been linked to health concerns. However, don’t hang up your frying pan just yet! There are ways to make frying healthier. Some clever folks discovered that adding antioxidants to the oil can help protect those unsaturated fats and keep them from transforming into less desirable forms. Plus, choosing oils that are naturally high in unsaturated fats, like olive oil or canola oil, can also reduce the formation of saturated and trans fats.
Key Takeaways:
- The Maillard reaction, triggered by high heat, is responsible for the desirable color and flavor of fried foods.
- The type of oil used in frying impacts the chemical composition of the food.
- Unsaturated fats can transform into saturated and trans fats during frying, potentially impacting health.
- Choosing healthier oils and using antioxidants can mitigate the formation of unhealthy fats.
What Happens to Carbon Atoms When Burned?
So, we’re talking about burning stuff, right? Like, what happens to the carbon atoms in there? Well, picture this: you’ve got these carbon atoms just chilling out in something like wood or coal. When you light it up, they get a major wake-up call. It’s like a wild party where they meet up with oxygen from the air, and things get pretty heated – literally!
This whole shebang is called combustion, and it’s a pretty big deal. It’s how we get power for our homes, fuel for our cars, and even heat for our stoves. But let’s get back to those carbon atoms. When they collide with oxygen, they ditch their old buddies and form a new molecule called carbon dioxide, or CO2. You’ve probably heard of it; it’s what we exhale and what plants need to grow. This new bond between carbon and oxygen releases a burst of energy, which is what we feel as heat and see as light from the flames. Pretty neat, huh?
Now, here’s the thing: while combustion is super useful, burning stuff like coal and oil releases a whole bunch of CO2 into the atmosphere. And too much CO2 can mess with the planet’s temperature, leading to climate change.
Scientists are hard at work trying to figure out ways to burn things more cleanly or find new sources of energy that don’t produce as much CO2. Think solar power, wind energy, even capturing carbon dioxide from power plants and storing it underground. It’s a complex issue with no easy answers, but understanding what happens to those tiny carbon atoms when we burn something is a good place to start!
Key Takeaways:
- Combustion is a chemical reaction between a substance and oxygen, releasing energy in the form of heat and light.
- Carbon atoms, present in fuels like wood and coal, react with oxygen during combustion to form carbon dioxide (CO2).
- While essential for energy production, the burning of fossil fuels is a major contributor to CO2 emissions and climate change.
- Scientists are actively researching cleaner combustion methods and alternative energy sources to mitigate these environmental impacts.
What Happens to Carbon When Eaten?
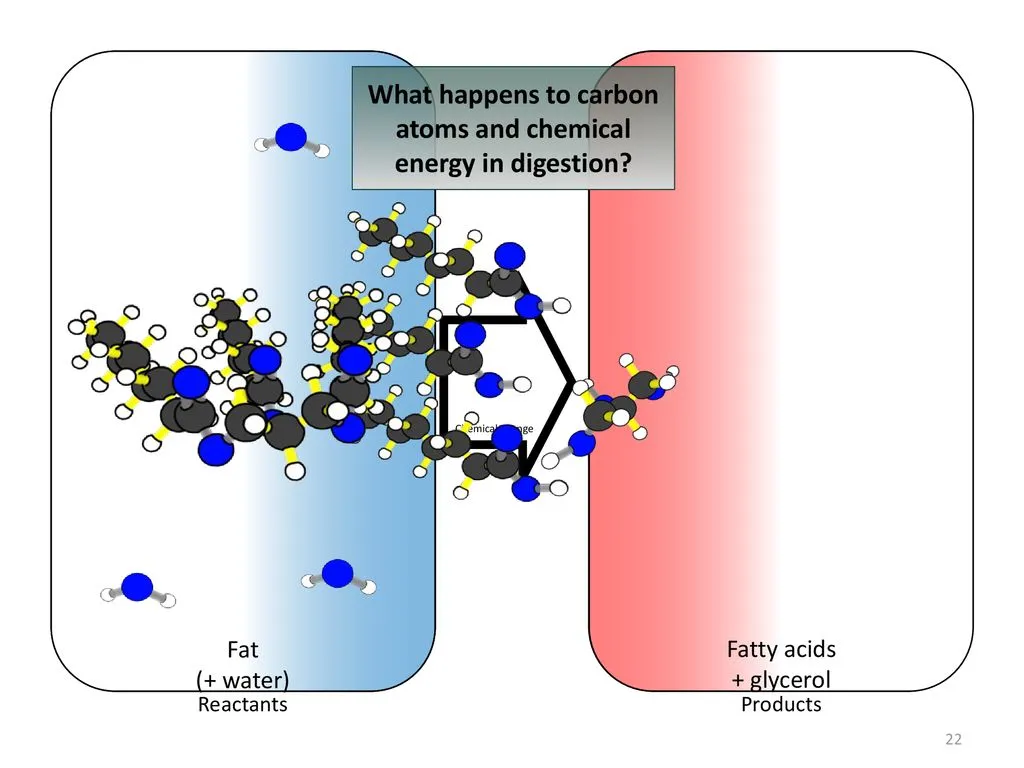
So, we’ve talked about how we get carbon from food. But what actually happens to those tiny carbon atoms once they’re inside us? Well, your digestive system is like a microscopic kitchen, breaking down the food you eat into smaller and smaller pieces. These pieces, containing our little carbon friends, are then absorbed into your bloodstream – think of it as a network of tiny delivery trucks – and whisked away to your body’s cells.
Now, at the cellular level, things get really interesting. Your cells are like mini factories, constantly building and rebuilding. They take those carbon atoms, delivered by the bloodstream, and use them as building blocks for all sorts of essential molecules like proteins, carbohydrates, and fats.
Think of it like this: you eat a delicious apple, full of carbon. Your body breaks it down, absorbs the carbon, and then uses those carbon atoms to build, say, a muscle protein that helps you lift heavy things. Pretty cool, right? But it doesn’t stop there! Any unused carbon can be stored in your body as fat – a reserve energy source for later. When you need an extra boost, your body can break down this stored fat, releasing those carbon atoms to fuel your activities.
Finally, remember that life is a cycle. Just like you breathe in oxygen and exhale carbon dioxide, the carbon atoms you consume eventually find their way back into the environment. This happens primarily through your breath and, well, let’s just say your bathroom breaks.
And what happens to those released carbon atoms? Some are taken up by plants during photosynthesis, starting the cycle anew. Others might end up in the soil, or even journey to the depths of the ocean. It’s a complex and fascinating process, and scientists are still unraveling all its mysteries. For example, did you know that researchers are studying how different types of food might influence the way our bodies process and store carbon? There’s still a lot to learn, but one thing is clear: every bite you take connects you to this incredible carbon cycle, linking you to the food you eat, the air you breathe, and the world around you.
Key Takeaways:
- Carbon atoms from food are absorbed into the bloodstream and transported to cells throughout the body.
- Cells utilize carbon as a fundamental building block to create essential molecules like proteins, carbohydrates, and fats.
- Excess carbon can be stored as fat, a reserve energy source for the body.
- Through respiration and decomposition, carbon atoms are released back into the environment, participating in a continuous cycle.
What Happens to Carbon When Something Dies?
So, we’ve talked about how carbon is a building block of life, right? It’s in everything from tiny bacteria to giant trees. But what happens to all that carbon when something dies? Well, it doesn’t just disappear! Instead, it embarks on a fascinating journey back into the environment.
Think of it like this: Imagine a tree that’s lived a long, happy life finally kicks the bucket. It falls to the forest floor, and that’s where the real action begins. Tiny organisms – mostly bacteria and fungi – move in and set up shop. They’re like nature’s recyclers. These decomposers break down the complex stuff in the tree – the wood, the leaves, you name it – and as they munch away, they release carbon dioxide back into the air.
But decomposition isn’t the only way carbon gets released. Remember respiration? That’s how we (and all living things) get our energy. We breathe in oxygen, and our bodies use it to break down food, releasing carbon dioxide as a byproduct. Well, guess what? Plants and animals do the same thing! So, even while something is alive, it’s constantly exchanging carbon with the environment through respiration. Now, here’s the cool part: the carbon dioxide released through decomposition and respiration doesn’t just float around aimlessly. Plants actually need it to survive! They absorb it from the air and use it during photosynthesis to create their own food (which, as a bonus, also releases oxygen back into the atmosphere – it’s a win-win!). Discover what happens to carbon atoms in photosynthesis.
So, you see, carbon is constantly cycling through the environment – moving from living things to the atmosphere, to the soil, and back again. It’s a delicate balance, and understanding how it works is super important, especially as we learn more about things like climate change.
Key Takeaways:
- Death initiates the decomposition process, where microorganisms break down organic matter, releasing carbon back into the environment.
- Respiration, essential for energy production in living organisms, also releases carbon dioxide.
- Plants play a crucial role in absorbing carbon dioxide during photosynthesis, utilizing it for growth and releasing oxygen.
- The carbon cycle highlights the continuous movement and transformation of carbon between living organisms and the environment.
What Happens to Carbon When Heated?
So, we’ve been talking about carbon, right? This amazing element that’s basically the foundation of, well, everything alive! But what happens when we crank up the heat on this essential building block? Let’s dive in and explore the wild world of hot carbon!
Think of carbon atoms like tiny dancers. When they get warm, they start switching partners and forming new bonds. At first, it’s a pretty simple dance – they pair up and create molecules like methane and ethane. But as the temperature climbs, the dance floor gets wilder! Those bonds break and reform, creating more complex structures like benzene and graphite. It’s like a molecular mosh pit!
Now, let’s talk about fire! You know how we burn things like wood for warmth or to cook? That’s combustion in action. Basically, carbon gets cozy with oxygen, and they release a ton of energy in the form of heat and light. This fiery tango is what powers many of our industries and keeps our homes warm. But there’s a catch – this process also creates carbon dioxide (CO2), a greenhouse gas that’s a major player in climate change.
But wait, there’s more! Under extreme heat, carbon atoms can completely transform themselves. They can rearrange into two very different forms: graphite and diamond. You probably know graphite – it’s what makes pencils write! It’s soft and slippery because of its layered structure, which also makes it great at conducting electricity. Then there’s diamond, the superstar of the carbon world. This dazzling gem is incredibly strong and tough because its atoms are bound together in a super-tight network.
Ever wonder why fried food tastes so good? It’s not just the grease, I promise! It’s all thanks to a chemical reaction called the Maillard reaction. When you heat up food with proteins and sugars, like that crispy chicken or those golden-brown fries, carbon gets in on the action. This complex reaction creates a cascade of new compounds that give fried food its signature color and that irresistible savory flavor.
Here’s the thing about science – we’re always learning! What we know about carbon and its reactions is constantly evolving. Researchers are still uncovering new details about how carbon behaves under different conditions. So, while we’ve covered some of the basics, there’s a whole universe of carbon chemistry waiting to be explored!
Key Takeaways:
- Heating carbon atoms causes them to become more reactive, forming new bonds and creating various molecules.
- Combustion, the process of burning, involves a reaction between carbon and oxygen, releasing energy as heat and light.
- Extreme heat can transform carbon into different allotropes, such as graphite and diamond, each with unique properties.
- The Maillard reaction, responsible for the browning and flavor of cooked foods, involves complex chemical reactions involving carbon.
- Ongoing research continues to unravel the complexities of carbon chemistry and its implications.
How are Carbon Atoms Destroyed?
So, we’ve talked about how carbon atoms are like the busy bees of the universe, constantly buzzing around from one form to another. But what happens when it’s time for these tiny particles to meet their maker? Can they actually be destroyed? The short answer is: not really. Here’s why.
Think of it like this: Imagine a Lego castle. You can break it down, rearrange the blocks, and even build something totally different, but the Legos themselves don’t just disappear, right? Carbon atoms are kind of like those Legos. They’re the fundamental building blocks of, well, practically everything! From the air we breathe to the food we eat, and even our own bodies.
When we talk about “destroying” carbon, what we’re really talking about is changing its form. It’s all about breaking and forming bonds – those tiny connections that hold atoms together. Let’s take that crispy fried treat example from earlier. When you’re frying up something delicious, the heat causes the carbon atoms in the food to break apart from their original arrangements. They then quickly latch onto other atoms nearby, like oxygen or hydrogen, forming new molecules. These new molecules are what give fried food its irresistible smell, taste, and that crispy golden-brown color. But notice, the carbon atoms themselves haven’t vanished, they’ve just gone through a bit of a makeover.
Now, let’s crank up the heat and talk about combustion. This is where things get a little more dramatic. Imagine a roaring bonfire. As the wood burns, the carbon atoms inside react with oxygen in the air. This fiery tango releases energy in the form of heat and light, and – here’s the important part – it also releases carbon dioxide into the atmosphere.
Again, the carbon atoms haven’t been obliterated, they’ve simply partnered up with oxygen. This is a big deal because carbon dioxide is a greenhouse gas, which means it traps heat and contributes to climate change. But wait, there’s more! Remember how we said carbon is the backbone of life itself? Well, when living things die and decompose, or when we exhale, carbon atoms are released back into the environment. It’s a continuous cycle, with carbon atoms constantly being recycled and repurposed.
Scientists are still unraveling all the complexities of this carbon cycle, but one thing is clear: completely destroying a carbon atom is a pretty tough thing to do. It takes an incredible amount of energy, the kind you’d find in the heart of a star or during a nuclear reaction. So, the next time you see a piece of wood burning or enjoy a plate of french fries, remember: the carbon atoms within are just embarking on a new adventure, transforming and interacting in ways we are still working to fully comprehend. It’s a fascinating journey that highlights the interconnectedness of our universe, from the smallest atom to the largest star.
Key Takeaways:
- Carbon atoms are not easily destroyed; they primarily undergo transformations, changing their form and bonding arrangements.
- Processes like frying and combustion involve the breaking and forming of chemical bonds, rearranging carbon atoms into new molecules.
- While carbon dioxide, a greenhouse gas, is released during combustion, the carbon atoms themselves are not destroyed, but rather incorporated into a new molecule.
- The carbon cycle demonstrates the continuous recycling and repurposing of carbon atoms through various natural processes.
- Complete destruction of carbon atoms requires immense energy levels, such as those found in stars or nuclear reactions.
Why is Carbon Left After Burning?
So, we’ve talked about how things burn, right? But have you ever noticed that there’s often some stuff left over after something burns? Like, think about a campfire – you’re left with ashes, right? And if you’ve ever seen a candle that was blown out too soon, you know it leaves behind black soot. That, my friends, is often carbon!
You see, burning is basically a chemical tango between whatever’s being burned and oxygen. They get together, react, and boom! – you get new stuff, like carbon dioxide, which is a gas. But here’s the thing: sometimes, there isn’t enough oxygen to go around for a complete party. And when that happens, we get what’s called incomplete combustion.
Think of it like this: imagine you’re baking cookies, but you only put in half the sugar the recipe calls for. Your cookies will still exist, but they won’t be quite right – they’ll be missing something. It’s the same with burning! If there’s not enough oxygen, the carbon in whatever’s being burned can’t fully combine with it to make carbon dioxide. Instead, it gets left behind as soot, ash, or charcoal.
Now, here’s where things get really interesting. This leftover carbon actually plays a big role in something called the carbon cycle. You know how we’re always hearing about the environment and climate change? Well, the carbon cycle is a major player in all that.
For instance, when forests burn in wildfires (which can be a natural part of the ecosystem, by the way), they release a whole bunch of carbon dioxide into the air. But on the flip side, plants are amazing little things – they actually suck up carbon dioxide from the atmosphere and use it to grow! It’s a delicate balance, and scientists are still trying to figure out all the intricacies of how it works.
One thing we do know for sure is that even though carbon can transform in all sorts of ways – from the food we eat to the fuel we burn in our cars – the total amount of carbon on Earth stays pretty much the same. It’s like a giant game of musical chairs, with carbon atoms constantly shifting from one form to another.
There’s still a lot we’re learning about carbon and its role in the universe, but one thing’s for sure: it’s a pretty important element, and understanding how it behaves is crucial for tackling some of the biggest challenges facing our planet today.
Key Takeaways:
- Incomplete combustion, due to insufficient oxygen, can result in carbon being left behind as soot, ash, or charcoal.
- This leftover carbon plays a role in the carbon cycle, a complex system involving the exchange of carbon between the atmosphere, oceans, land, and living organisms.
- Wildfires, while a natural part of some ecosystems, release significant amounts of carbon dioxide into the atmosphere, impacting the carbon cycle.
- Plants act as carbon sinks, absorbing carbon dioxide during photosynthesis, highlighting the interconnectedness of natural processes.
- While carbon undergoes various transformations, the total amount on Earth remains relatively constant, emphasizing the importance of understanding its movement and balance.
What Happens to Carbon After a Fire?
So, we’ve been talking about fire and stuff, right? One thing you might not have thought about is what happens to all the carbon in a fire. We know fire needs oxygen, but what’s the deal with carbon?
Think of it like this: imagine tiny carbon atoms hanging out in trees and plants, just doing their thing. Then, whoosh – a fire starts! Suddenly, these carbon atoms get really heated – literally! This heat gives them the energy to break free and hook up with oxygen in the air. When carbon and oxygen get together like that, they form a new gas called carbon dioxide. Ever heard of that one?
Now, if the fire is super intense and has plenty of oxygen to work with – we’re talking a real bonfire here – most of the carbon atoms will find themselves an oxygen buddy. This is called “complete combustion”, and it basically means everything burns up as cleanly as possible, turning into carbon dioxide and water vapor.
But here’s the catch: sometimes, a fire doesn’t have enough oxygen to go around. Maybe there’s just too much stuff burning at once, or the wind isn’t cooperating. In this case, things get a little messier. Instead of forming carbon dioxide, some of the carbon atoms might only partially link up with oxygen. They might even cling to other stuff floating around in the smoke and ash. This is called “incomplete combustion” and it’s why you sometimes see dark, sooty smoke – that’s basically unburned carbon escaping into the air.
Speaking of smoke, let’s talk about wildfires for a second. You know, those massive blazes that make the news every summer? Yeah, those have a huge impact on carbon. See, when a wildfire rips through a forest, it releases tons of carbon dioxide into the atmosphere. This is because all the trees and plants that get burned up release their stored carbon as they turn to ash.
Now, scientists are always studying this stuff because it’s super important for understanding climate change and how carbon moves around our planet. Some experts believe that wildfires might be getting more intense and happening more often because of things like climate change itself (kind of ironic, right?). The more carbon dioxide in the atmosphere, the warmer our planet gets, and the drier things become, making them more likely to catch fire. It’s a bit of a vicious cycle.
Anyway, the main takeaway here is that fire transforms carbon. Sometimes it turns into carbon dioxide, which is a big deal for our climate. Other times, it forms soot and other particles that can pollute the air. Scientists are still learning about all the different ways that carbon behaves in fires, and their research helps us understand how to manage forests, fight climate change, and keep our air clean.
Key Takeaways:
- During a fire, carbon atoms react with oxygen, primarily forming carbon dioxide (CO2).
- Complete combustion occurs with sufficient oxygen, producing CO2 and water vapor.
- Incomplete combustion, due to limited oxygen, results in the release of soot, carbon monoxide, and other byproducts.
- Wildfires significantly impact the carbon cycle, releasing large amounts of CO2 into the atmosphere.
- The intensity and frequency of wildfires are potentially influenced by climate change, leading to a concerning feedback loop.
- Understanding carbon’s behavior in fires is crucial for forest management, climate change mitigation, and air quality control.
What Does Burning Do to Carbon?
So, we’ve been talking about what happens when things burn. Think of it like this: carbon atoms are having a wild party with oxygen. They get so excited that they release energy in the form of heat and light – that’s the fire we see! They’re basically doing a little dance, rearranging themselves to form a new molecule called carbon dioxide. Picture it like this: the carbon and oxygen hold hands, spin around, and poof! They turn into carbon dioxide, releasing a bit of water vapor along the way.
But here’s the thing: sometimes, this “dance party” can be a bit chaotic. If there’s plenty of oxygen to go around, the carbon and oxygen pair up perfectly, and everything’s cool. We call this complete combustion. It’s like a well-choreographed waltz.
But what if there isn’t enough oxygen? Well, things get a little messy. Instead of a neat waltz, we get an awkward shuffle, and some carbon atoms are left hanging without an oxygen partner. This is incomplete combustion, and it leaves behind traces of soot and carbon monoxide, kind of like leftover party decorations.
Now, scientists are always trying to understand these processes better. There are still questions about how different factors, like temperature or the type of fuel, affect combustion. And because combustion plays a big role in things like power plants and car engines, figuring out how to make it cleaner and more efficient is super important for the environment. So, while we’ve got a good handle on the basics, there’s always more to learn about the fascinating world of carbon and its fiery dance with oxygen!
Key Takeaways:
- Burning, or combustion, is a chemical reaction between carbon and oxygen, releasing heat and light.
- Complete combustion occurs with ample oxygen, producing carbon dioxide and water vapor.
- Incomplete combustion, due to insufficient oxygen, results in the formation of soot, carbon monoxide, and other byproducts.
- Understanding and improving combustion efficiency is crucial for reducing emissions and environmental impact.
What Happens When Carbon Compounds Are Burnt?
So, we’ve been talking about burning stuff, specifically things made up of carbon. You know, like wood, paper, or even the gas in your car. When these things burn, they’re actually going through a pretty important chemical reaction called combustion. Let’s break it down a bit further, shall we?
Think of it like this: imagine you’ve got a campfire going. You toss a log on there, and what happens? The wood starts to disappear as flames dance around it, right? That’s because the carbon in the wood is reacting with the oxygen in the air.
Now, this reaction isn’t just for show; it actually releases a bunch of energy in the form of heat and light. That’s why a fire can keep you warm on a chilly night! This energy release is what makes combustion so useful for things like generating electricity or powering engines.
But here’s the catch: this reaction doesn’t just magically make the wood disappear. It actually produces something new: carbon dioxide, which you might know by its chemical abbreviation, CO2. You see, the carbon atoms in the wood combine with the oxygen in the air, and voila, you get CO2.
Now, there are times when this burning process doesn’t go quite as planned. Imagine trying to start a fire with damp wood. It might smolder and smoke a lot more, right? That’s because there isn’t enough oxygen for all the carbon to turn into CO2. This is called incomplete combustion, and it can produce other things besides CO2, like carbon monoxide (CO), which is pretty nasty stuff.
Here’s where things get interesting: scientists are still studying all the ins and outs of combustion. We know the basics, but there are always new discoveries being made, especially when it comes to finding cleaner and more efficient ways to burn fuel. For example, some researchers are exploring ways to capture the CO2 produced during combustion, preventing it from going into the atmosphere. This is a big deal because CO2 is one of the main culprits behind climate change.
So, the next time you strike a match or turn on a gas stove, remember the amazing chemical reaction taking place: combustion. It’s a powerful process with a fascinating history, and its future is still being written!
Key Takeaways:
- Combustion of carbon compounds, such as wood or fuel, involves a reaction with oxygen, releasing energy as heat and light.
- This reaction primarily produces carbon dioxide (CO2), a greenhouse gas.
- Incomplete combustion, due to insufficient oxygen, can produce harmful byproducts like carbon monoxide (CO).
- Research continues to focus on developing cleaner and more efficient combustion technologies to mitigate environmental impact.
What Happens to Carbon After Combustion?
So, we were talking about how carbon does this whole song and dance when we’re frying up some deliciousness, right? Think of it like this: as things heat up, those tiny carbon atoms we can’t even see get really energized. They’re like, “Party time!” and start looking to mingle.
Now, oxygen in the air is always down for a good time, and it’s got a thing for carbon. They’re practically inseparable on the dance floor. When they link up, it’s explosive! They release a burst of energy in the form of heat and light, and voila, you’ve got combustion happening. This is what makes fire hot and glowy – it’s those carbon and oxygen atoms having a blast!
The main outcome of this wild party? Carbon dioxide, or CO2, which floats off into the air. It’s like the little souvenir left behind from the carbon and oxygen get-together. Now, if there’s plenty of oxygen to go around, all the carbon gets paired up, and we call that “complete combustion.”
But sometimes, there’s not enough oxygen for every carbon atom. It’s like showing up to a party and realizing there aren’t enough dance partners! In that case, you get “incomplete combustion,” and things get a little messy. Instead of just CO2, you might end up with carbon monoxide (CO), which is a bit of a party pooper because it’s actually toxic. Think of it like the unwanted guest who crashes the party and causes trouble.
And then there’s soot, those black, powdery remnants you sometimes see after a fire. That’s just leftover carbon that didn’t manage to find an oxygen partner. It’s like the wallflower carbon that didn’t get a chance to dance.
Now, this whole combustion thing is super important, even beyond our kitchens. Think about cars, power plants, and all those things that use fuel. They rely on combustion to work. The problem is, burning stuff like fossil fuels releases a lot of CO2 into the atmosphere. And remember how CO2 is a byproduct of that carbon-oxygen party? Well, too much of it in the atmosphere leads to a warming effect on the planet, which is what we call climate change – a whole other party we definitely don’t want to be a part of!
Scientists are working hard to find ways to generate energy without relying so much on burning fossil fuels. It’s like trying to find a new dance move that doesn’t involve carbon and oxygen going wild! There are some promising leads, like solar and wind power, but it’s an ongoing process.
So, the next time you’re cooking something delicious and see a little flame, remember the carbon-oxygen party going on at a microscopic level. It’s a fascinating process that powers our world but also comes with some big responsibilities.
Key Takeaways:
- Combustion involves a reaction between carbon and oxygen, releasing energy, carbon dioxide (CO2), and water vapor.
- Complete combustion occurs with ample oxygen, resulting in CO2 as the primary product.
- Incomplete combustion, due to limited oxygen, can produce carbon monoxide (CO) and soot.
- Excessive CO2 emissions from burning fossil fuels contribute to climate change.
- Finding alternative energy sources and improving combustion efficiency are crucial for reducing environmental impact.
- Unlocking Francis Alexander Shields’ Finance Empire: A Comprehensive Biography - July 12, 2025
- Unveiling Francis Alexander Shields: A Business Legacy - July 12, 2025
- Francis Alexander Shields’ Business Career: A Comprehensive Overview - July 12, 2025















1 thought on “The Carbon Tango: Unveiling the Science of Frying”
Comments are closed.