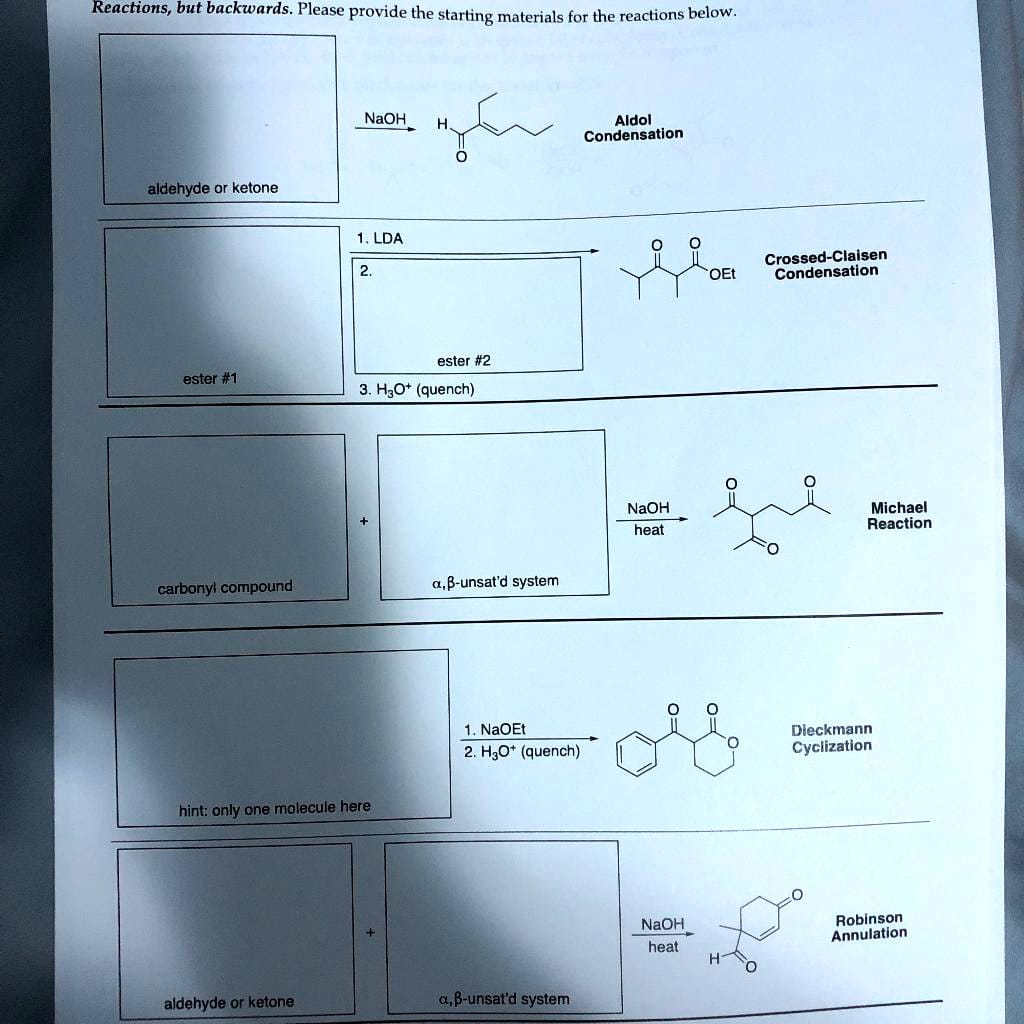
The Robinson Annulation, a cornerstone of organic synthesis, is a powerful reaction that constructs six-membered rings by uniting an enolate and an α,β-unsaturated carbonyl compound. This elegant process, a tandem Michael addition-aldol condensation sequence, empowers chemists to synthesize complex cyclic molecules crucial in pharmaceuticals, natural products, and materials science. This article provides a comprehensive overview of the reaction, covering its essential components, mechanism, applications, and recent advances.
Understanding the Key Reactants
The Robinson Annulation hinges on the interplay of two crucial reactants: the enolate and the α,β-unsaturated carbonyl compound. Retro-synthetically analyzing the product’s α,β-unsaturated ketone reveals these required starting materials. Let’s delve into each component:
Enolates: The Nucleophilic Partner
Enolates, formed by the deprotonation of a carbonyl compound (ketone or aldehyde) at the α-carbon, are electron-rich and act as nucleophiles. This negative charge density at the α-carbon makes them eager to react with electron-deficient species. The choice of base used for deprotonation can influence the reaction’s regioselectivity and overall efficiency.
α,β-Unsaturated Carbonyl Compounds: The Electrophilic Partner
α,β-Unsaturated carbonyl compounds possess a C=C double bond conjugated to a carbonyl group (C=O). This conjugation creates an electron-deficient β-carbon, making it susceptible to nucleophilic attack. The nature of the carbonyl group (aldehyde or ketone) and any additional substituents can significantly impact the reaction’s outcome.
Deconstructing the Mechanism
The Robinson Annulation proceeds through a two-step mechanism: Michael addition followed by intramolecular aldol condensation.
Step 1: Michael Addition
The enolate initiates the reaction by attacking the β-carbon of the α,β-unsaturated carbonyl compound in a Michael addition. This forms a new C-C bond and creates an enolate intermediate.
Step 2: Intramolecular Aldol Condensation
The newly formed enolate intermediate then undergoes an intramolecular aldol condensation. This step involves the nucleophilic attack of the enolate oxygen onto the carbonyl carbon, ultimately forming a six-membered ring containing an α,β-unsaturated ketone.
Retro-Synthetic Analysis: Working Backwards
A powerful tool for planning a Robinson Annulation is retro-synthetic analysis. By cleaving the C=C bond of the target molecule’s α,β-unsaturated ketone and the adjacent C-C bond within the ring, you can identify the required enolate and α,β-unsaturated carbonyl starting materials.
Reaction Conditions and Optimization
Optimizing reaction conditions, such as base selection and temperature, is crucial for controlling regio- and stereoselectivity in Robinson Annulations. Factors like solvent, reaction time, and the use of additives can also influence the yield and purity of the desired product. Ongoing research continues to explore new catalysts and optimized conditions to enhance the efficiency and selectivity of this reaction.
Applications and Significance
The Robinson Annulation is a versatile tool with broad applications in various fields, including:
- Pharmaceutical Synthesis: Constructing complex ring systems found in many drugs and therapeutic agents.
- Natural Product Synthesis: Mimicking biosynthetic pathways to access valuable natural compounds.
- Materials Science: Creating novel materials with tailored properties.
Exploring Advanced Concepts
Beyond the basics, the Robinson Annulation encompasses several intricate aspects that continue to be areas of active research:
Stereochemistry
The reaction can generate multiple stereoisomers, and controlling the stereochemical outcome is a significant challenge. Researchers are exploring chiral catalysts and other strategies to achieve diastereoselective and enantioselective Robinson Annulations.
Variations and Modifications
Several modifications to the classic Robinson Annulation exist, expanding its synthetic utility. These include variations in the electrophilic partner (e.g., using nitroalkenes) and modifications like the Wichterle reaction employing methyl vinyl ketone. There is ongoing research in this domain to broaden the scope of applicable substrates and improve reaction efficiency.
Limitations and Challenges
While powerful, the Robinson Annulation has some limitations. Certain starting materials may lead to unwanted side reactions like polymerization. Careful selection of reactants and optimization of reaction conditions are essential to mitigate these challenges. Researchers are also actively investigating milder reaction conditions and more tolerant catalysts to address these limitations.
Conclusion
The Robinson Annulation remains a vital reaction in organic chemistry, providing access to a wide range of complex cyclic structures. Mastering this reaction empowers chemists to design and synthesize molecules with important biological activities and material properties. While the fundamental principles are well-understood, ongoing research continues to refine our understanding of its intricacies and expand its applications. For a detailed overview of the Victor Valley College area and its landmarks, explore the interactive VVC map. Information on the AFLS assessment process can be found here.
- Jerry McSorley’s Post-Divorce Life: New Beginnings - July 16, 2025
- The Rise and Fall of the New Haven Nighthawks: A Minor League Hockey Legacy - July 16, 2025
- Unlock Jerry McSorley’s Career Highlights: Eye Tax Inc.’s Solar Success - July 16, 2025