This guide provides a comprehensive overview of titration, a crucial chemical analysis technique. We’ll explore the roles of titrant and titrator, delve into different titration types, and discuss the evolution and future of this essential analytical method.
Decoding Titration Basics
Titration, also known as titrimetry and volumetric analysis, is a laboratory method used to determine the concentration of an unknown substance (the analyte) by reacting it with a solution of known concentration (the titrant). It’s a fundamental technique in chemistry with applications across numerous scientific and industrial fields. If you’re interested in learning more about chemical reactions, take a look at the science balancing equations worksheet.
Titration Components: The Key Players
At the heart of every titration are three key components:
- The Titrant: This is the solution of known concentration, acting as the measuring tool. Its precise concentration is critical for accurate analysis.
- The Titrand (or Analyte): This is the substance being analyzed, the “mystery” solution whose concentration we’re trying to determine.
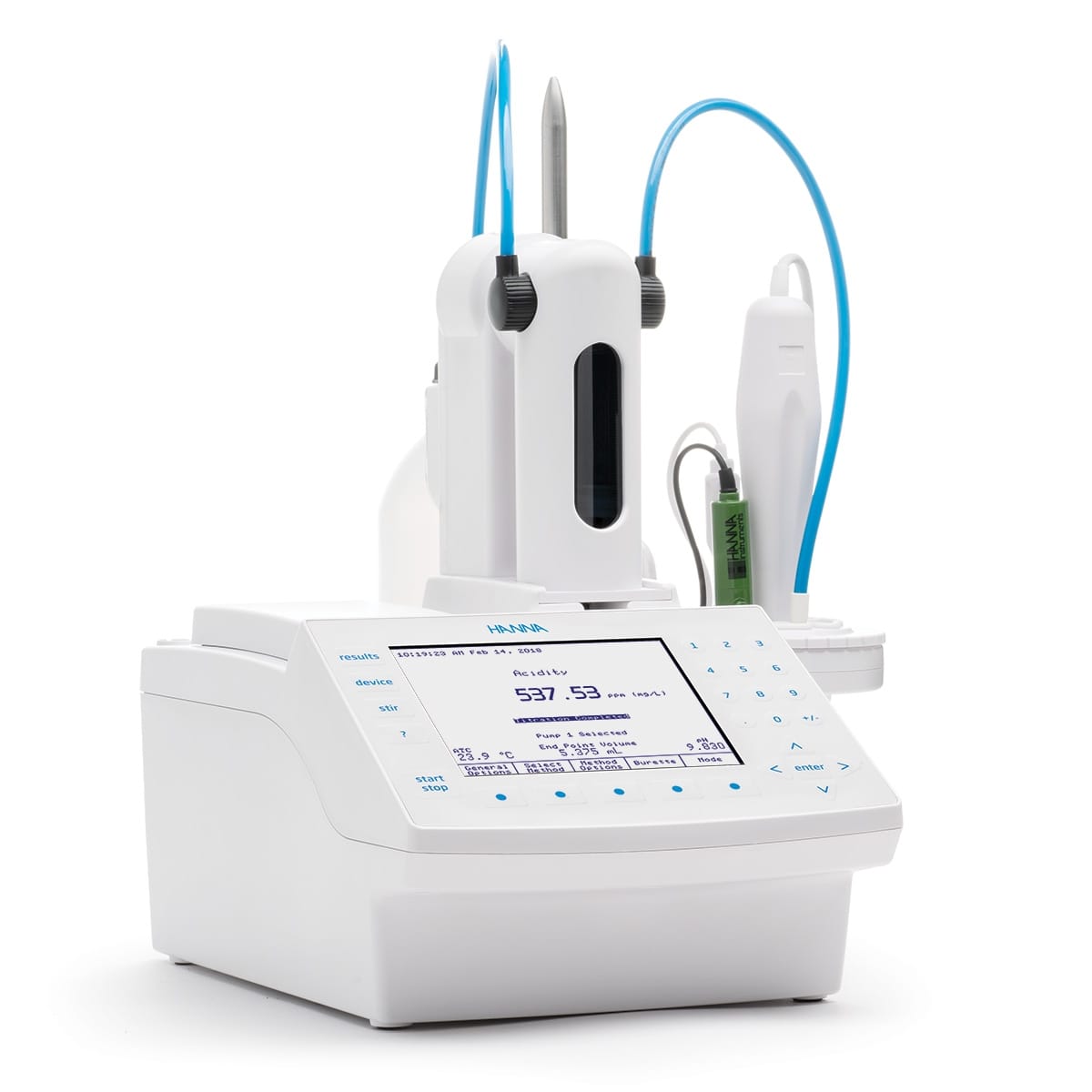
- The Titrator: This can refer to the person performing the titration or the instrument used to deliver the titrant (such as a burette or an automated titration system).
Think of it like baking: the titrant is your carefully measured sugar, the titrand is your cake batter, and the titrator is you, carefully adding the sugar.
The Titre: Measuring the Reaction
The titre is the precise volume of titrant required to reach the equivalence point—where the analyte and titrant have perfectly reacted. This carefully measured volume is what allows us to calculate the unknown concentration of the analyte. It’s like noting exactly how much sugar you added to achieve the perfect cake sweetness.
Exploring Different Titration Types
Just as a chef uses different tools for different dishes, chemists utilize various titration types, each suited to specific analytical challenges:
- Acid-Base Titration: The most common type, using a reaction between an acid and a base to determine the concentration of one of them.
- Redox Titration: This type focuses on electron transfers, where one substance is oxidized and another is reduced. This helps determine the concentration of substances involved in these redox reactions.
- Complexometric Titration: This involves forming a complex between a metal ion and a complexing agent, allowing for the determination of metal ion concentrations.
- Precipitation Titration: Two solutions react to form a solid precipitate. The amount of precipitate formed allows us to determine the reactant concentration.
- Karl Fischer Titration: A specialized titration that specifically measures water content in a sample. It’s invaluable in industries where even trace amounts of water can significantly impact quality.
Titrators: From Manual to Modern
Titrators have evolved significantly, from manual to automated systems:
- Manual Burettes: These classic tools provide hands-on control over titrant addition but require skill and careful attention to detail.
- Automatic Titrators: These sophisticated systems offer increased precision, speed, and automation. They handle titrant delivery, endpoint detection, and calculations.
Titration in Action: Real-World Applications
Titration plays a crucial role in diverse industries:
- Pharmaceuticals: Ensuring drug purity and accurate dosage.
- Environmental Monitoring: Measuring pollutants and assessing water quality.
- Food Industry: Determining acidity and pH levels in food and beverages.
- Biodiesel Production: Measuring and neutralizing acidity in oils.
- Virology: Understanding virus infectivity. If you’re interested in learning about alternative energy sources, click on the solar panel teas passage.
The Future of Titration
The field of titration continues to advance, with ongoing research focusing on:
- New Titrant Chemistries: Exploring new reagents for increased selectivity and sensitivity.
- Miniaturization: Developing smaller titrators for micro-scale analysis.
- Integration with Other Techniques: Combining titration with other analytical methods for more comprehensive analyses.
While advancements continue, it’s important to acknowledge that titrations are susceptible to certain challenges. For example, the choice of indicator can introduce error, and some reactions may proceed slowly. Ongoing research suggests that automated titrators and novel indicator chemistries might address these limitations. This research also suggests that our understanding of titration nuances will probably become more refined over time.
Titration remains a cornerstone of chemical analysis, essential for understanding and quantifying the world around us. As technology evolves, we can expect this vital technique to become even more precise, efficient, and versatile.
- Discover Long Black Pepper: Flavor & Health Benefits - April 25, 2025
- Shocking Twists: The Grownup Review: Unreliable Narration - April 25, 2025
- A Quiet Place Book vs Movie: A Deep Dive - April 25, 2025
















1 thought on “Understanding Titrant and Titrator: A Comprehensive Guide to Modern Titration”
Comments are closed.