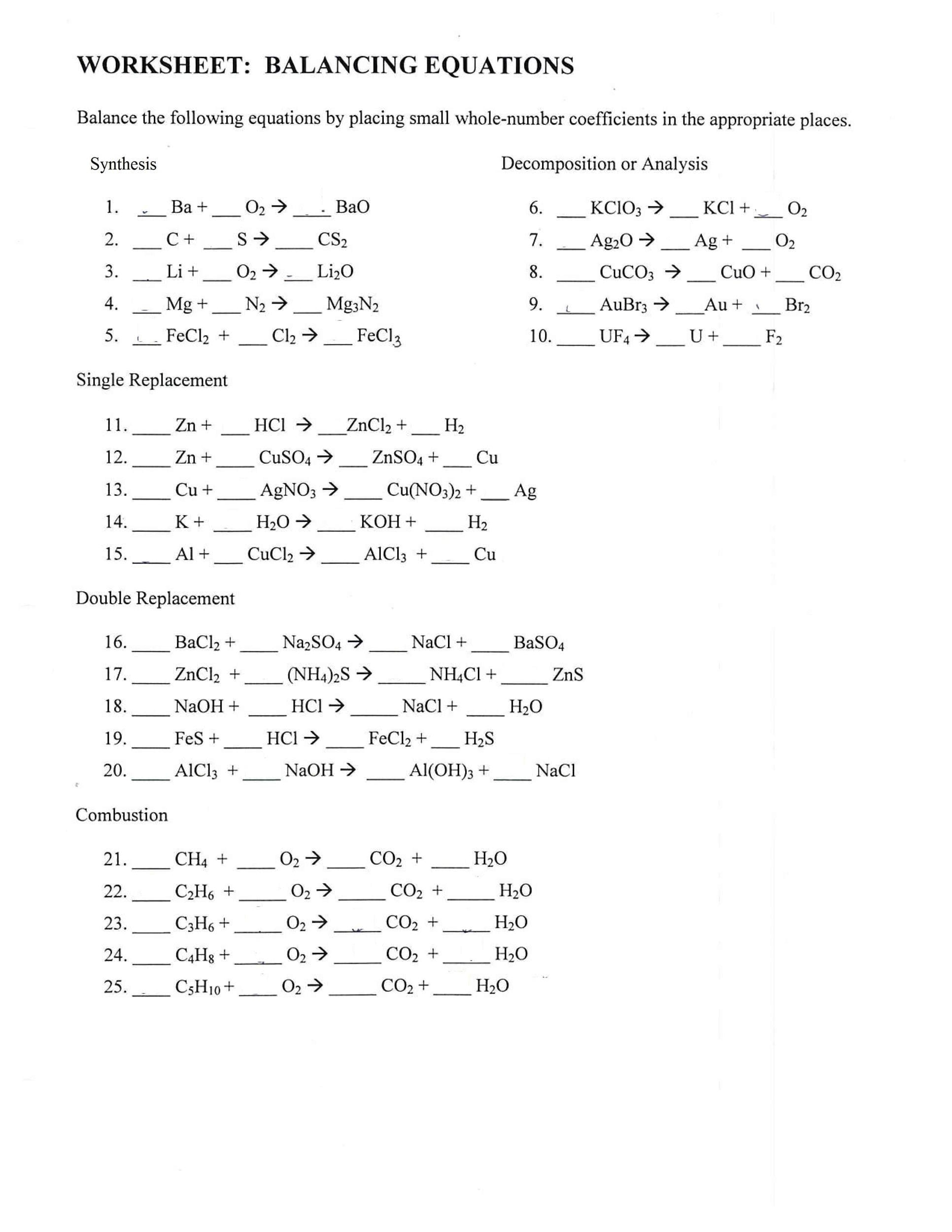
Mastering chemical equations is like unlocking a secret code to the universe. This guide provides the keys to understanding and balancing these equations, offering a comprehensive journey from basic concepts to advanced techniques, complete with free printable worksheets, engaging videos, and practical real-world applications.
Deciphering Chemical Equations
Let’s break down the basics. A chemical equation is essentially a recipe for a chemical reaction. It tells us what ingredients we start with (reactants) and what we end up with (products). Just like a recipe needs precise measurements, a chemical equation needs to be balanced. This ensures that the number of atoms of each element is the same on both sides of the equation, reflecting the fundamental Law of Conservation of Mass – matter can neither be created nor destroyed in a chemical reaction.
This concept, known as stoichiometry, is the bedrock of chemistry. It allows us to predict the amounts of reactants and products involved in a reaction. Explore the interactive tool for creating stem-and-leaf displays conveniently using the stem and leaf display calculator. Mastering stoichiometry through balancing equations opens doors to understanding how the world around us works, from the smallest atoms to the largest stars.
Balancing Act: A Step-by-Step Guide
Balancing a chemical equation can feel like solving a puzzle, but with a systematic approach, it becomes straightforward. Here’s a step-by-step method:
Write the Unbalanced Equation: Begin with the basic chemical formulas of the reactants and products. This is your starting point.
Atomic Inventory: List each element present on both sides of the equation and count how many atoms of each you have. A table can be helpful for organization.
Start Simple, Balance One Element: Choose an element that appears in only one reactant and one product. Adjust the coefficients (the numbers in front of the formulas) to balance this element. Remember, never change subscripts (the small numbers within the formulas)—this alters the chemical itself.
Tackle the Rest: Systematically balance the remaining elements, one by one. You may need to revisit and adjust coefficients as you progress.
Simplify: If all coefficients share a common factor, divide them to achieve the smallest possible whole numbers.
Double-Check: Verify your work by recounting the atoms on both sides. Accuracy is key!
Why Balancing Matters
Balancing equations is more than just a classroom exercise; it’s fundamental to understanding the world around us:
- Stoichiometry Mastery: Predict the quantities of reactants and products in chemical reactions.
- Foundation for Advanced Chemistry: Provides a basis for more complex concepts and calculations.
- Critical Thinking Boost: Enhances problem-solving and analytical skills.
- Exam Preparation: Equips you to confidently tackle chemistry assessments.
- Real-World Connections: Understand the role of balanced equations in environmental science, medicine, industrial chemistry, and more.
Unveiling Reaction Types
Chemical reactions come in various flavors, each with its own balancing nuances:
- Synthesis: Simple components combine to form a more complex substance.
- Decomposition: A complex substance breaks down into simpler components.
- Single Displacement: One element replaces another in a compound.
- Double Displacement: Two compounds exchange elements or ions.
- Combustion: A substance reacts with oxygen, often producing heat and light.
Common Pitfalls and How to Avoid Them
Even seasoned chemists can stumble. Watch out for these common mistakes:
- Altering Subscripts: Changing subscripts changes the chemical identity—a major error!
- Overlooking Diatomic Elements: Remember that elements like hydrogen (H₂), oxygen (O₂), nitrogen (N₂), and halogens exist as diatomic molecules.
Practice Makes Perfect: Free Worksheets and Resources
Honing your balancing skills requires practice. Thankfully, a wealth of free resources is available:
- Printable Worksheets: Numerous websites offer downloadable PDFs with practice problems and answer keys, catering to various skill levels, from KS3/GCSE to advanced chemistry.
- Interactive Videos: Engaging video tutorials break down the balancing process step by step, making complex concepts easier to grasp.
- Online Simulators: Interactive simulations provide a dynamic learning experience, allowing you to experiment with different equations and visualize the reactions.
Explore resources like School Learning Resources, Science Notes and Projects, TemplateLab, Chemistrytutor.me, Chemrevise, Kapow Primary, ScienceWorksheets, Kentchemistry.com, and Sciencespot.net. Many helpful YouTube videos are also available from creators like Wayne Breslyn (Dr. B.), Cognito, The Organic Chemistry Tutor, and more.
By dedicating time to practice and utilizing these readily available resources, you’ll become a balancing equations expert in no time.
Beyond the Basics: Advanced Techniques and Future Directions
While the fundamental principles of balancing equations remain constant, ongoing research continues to deepen our understanding of chemical reactions, particularly under extreme conditions. This suggests that future discoveries may influence how we approach complex reactions. For those seeking a deeper dive, explore advanced balancing techniques such as using oxidation numbers or the algebraic method.
While current knowledge provides a strong foundation, the world of chemical reactions is dynamic and constantly evolving. By embracing continuous learning and exploring new perspectives, you can stay at the forefront of this fascinating field.
- Star Ring Trends: Etsy vs Amazon - March 28, 2025
- Boost Pollinator Habitats: Baby Blue Eyes Sustainable Farming Guide - March 28, 2025
- Protect Big Black Bears: Effective Conservation Strategies - March 28, 2025