Stuck on those tricky polyatomic ions? This interactive quiz and guide will help you conquer these essential chemical groups. We’ll break down what polyatomic ions are, highlight common ones, and offer clever memorization tips. Get ready to boost your chemistry knowledge!
What Are Polyatomic Ions?
Polyatomic ions are like tiny molecular squads with an electrical charge. Unlike single-atom ions (monatomic ions), these groups of atoms stick together and carry a positive or negative charge as a unit. This charge results from gaining or losing electrons. Understanding these ions is foundational to chemistry, helping us grasp how compounds behave and react. It also involves prefixes like hypo- and per- and suffixes like -ite and -ate that offer clues about their composition.
Why Do Polyatomic Ions Matter?
Polyatomic ions are key players in countless chemical compounds and reactions, influencing everything from the food we eat to the medicines we take. Understanding them unlocks a deeper understanding of the world around us.
Common Polyatomic Ions
Some polyatomic ions are ubiquitous. Familiarize yourself with these frequent flyers:
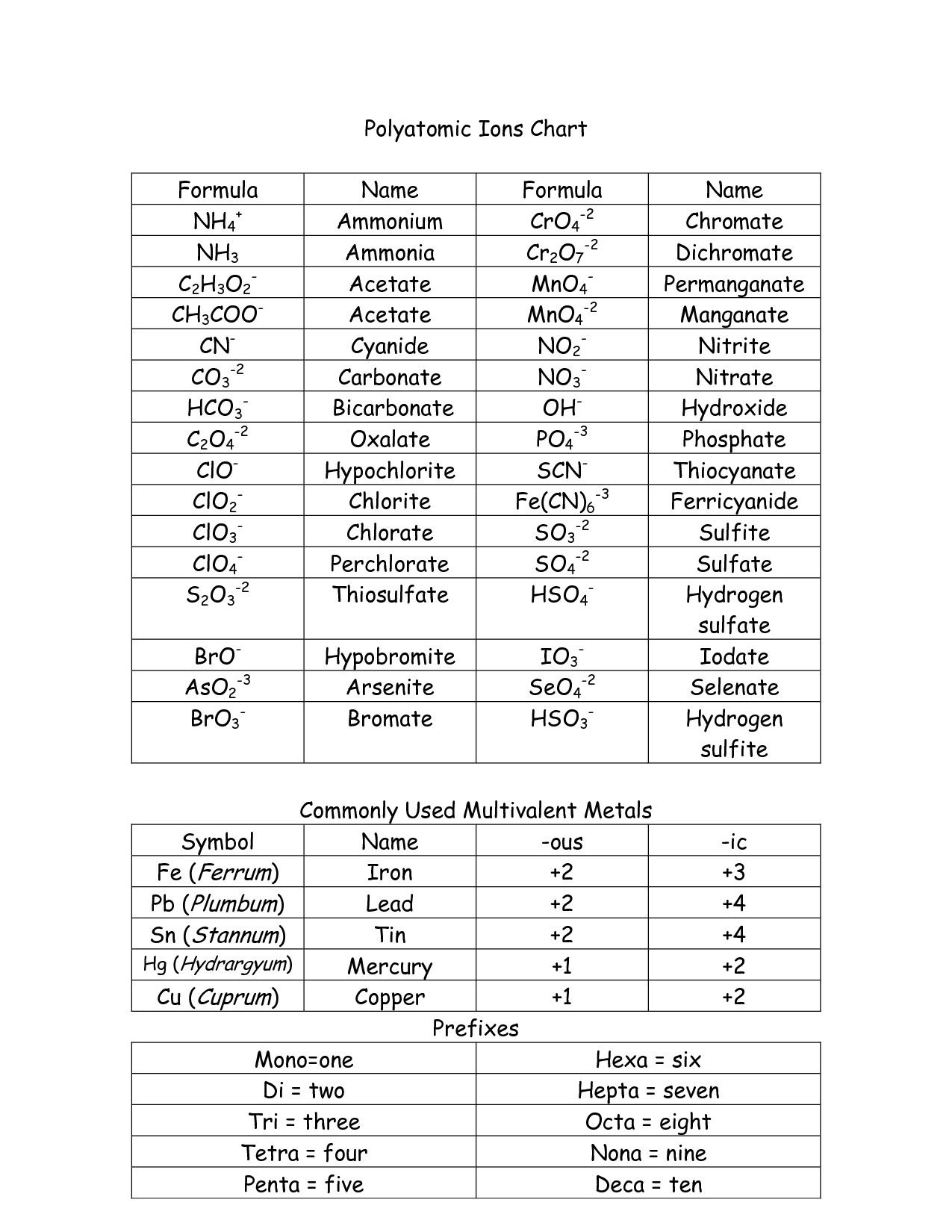
| Formula | Name | Charge |
|---|---|---|
| NH₄⁺ | Ammonium | +1 |
| OH⁻ | Hydroxide | -1 |
| NO₃⁻ | Nitrate | -1 |
| SO₄²⁻ | Sulfate | -2 |
| CO₃²⁻ | Carbonate | -2 |
| PO₄³⁻ | Phosphate | -3 |
This table isn’t exhaustive, but it’s a solid starting point. You’ll likely encounter these ions often. For a more complete list, check out our guide on Common Polyatomic Ions – Names, Formulas, and Charges.
Decoding the Names
Naming polyatomic ions follows some predictable patterns. Prefixes like hypo- and per- and suffixes like -ite and -ate provide hints about the number of oxygen atoms. This nomenclature is covered in more detail in our guide What is a Polyatomic Ion? – Definition and Importance.
Quiz Time!
What’s the formula for sulfate? Does ammonium have a positive or negative charge? Test your knowledge with our interactive quiz below! For beginner-level practice, try our Mastering Polyatomic Ions – Quiz 1 – Beginners.
(Interactive quiz would be placed here)
Memorization Hacks
Memorizing polyatomic ions doesn’t have to be a chore. Mnemonics, flashcards, writing repetitions, or even creating songs can make it more engaging. Experiment and find what works best for you. Consider exploring digital flashcard apps or online quiz platforms for additional practice.
Beyond the Basics
Once you’ve mastered the basics, why not explore further? Polyatomic ions play unique roles in various scientific fields, from environmental science to materials engineering. Want to dive deeper? Check out educational videos and other online resources to expand your knowledge. For a thought-provoking challenge outside of polyatomic ions, try the AP Brainrot Exam.
What Is a Polyatomic Ion? A Deeper Dive
A polyatomic ion is a charged chemical species composed of two or more covalently bonded atoms acting as a single unit. This charge arises from an imbalance of protons and electrons. Understanding polyatomic ions is crucial for grasping concepts like acid-base chemistry, salt formation, and chemical reactions. While “poly” implies many, even two atoms can form a polyatomic ion, highlighting their diverse nature. Mastering polyatomic ion nomenclature involves recognizing patterns related to prefixes, suffixes, and the oxidation state of the central atom.
Understanding Polyatomic Ion Formation
Polyatomic ions often form through protonation (adding H⁺) or deprotonation (removing H⁺) of a neutral molecule. This process is central to many chemical reactions.
Why Polyatomic Ions Matter: Real-World Examples
Polyatomic ions play vital roles in various fields:
- Environmental Science: Sulfate and nitrate ions contribute to acid rain.
- Medicine: Bicarbonate ions help regulate blood pH.
- Agriculture: Phosphate ions are crucial components of fertilizers.
Common Polyatomic Ions: An Extended List
| Charge | Cation (Positive) | Anion (Negative) |
|---|---|---|
| +1 | Ammonium (NH₄⁺) | Hydronium (H₃O⁺) |
| -1 | Hydroxide (OH⁻) | |
| Nitrate (NO₃⁻) | ||
| Cyanide (CN⁻) | ||
| Acetate (CH₃COO⁻) | ||
| Hypochlorite (ClO⁻) | ||
| Chlorite (ClO₂⁻) | ||
| Chlorate (ClO₃⁻) | ||
| Perchlorate (ClO₄⁻) | ||
| -2 | Sulfate (SO₄²⁻) | |
| Carbonate (CO₃²⁻) | ||
| Dichromate (Cr₂O₇²⁻) | ||
| -3 | Phosphate (PO₄³⁻) |
This expanded list provides a more comprehensive overview of common polyatomic ions. While research is ongoing, this information offers a solid foundation.
Common Polyatomic Ions: Names, Formulas, and Charges – In Detail
This section dives deeper into the world of polyatomic ions, focusing on their names, formulas, and charges. Recognizing these aspects is crucial for understanding chemical formulas, reactions, and the composition of compounds.
Decoding the Structure
Polyatomic ions are distinguished by their electrical charge. Neutral molecules have balanced positive and negative charges, while polyatomic ions have an overall charge, making them highly reactive. This charge dictates their interactions and the formation of diverse compounds.
Real-World Relevance
Polyatomic ions are ubiquitous in everyday life, from baking soda to fertilizers. Understanding them unlocks a deeper understanding of the world around us.
Naming Conventions
The “-ate” and “-ite” suffixes and prefixes like “per-” and “hypo-” provide clues about the oxygen content and charge. “-ate” typically signifies more oxygen atoms than “-ite” for a specific element. These subtle differences reflect important variations in their chemical behavior.
Charges and Interactions
Polyatomic ion charges dictate their interactions. Opposite charges attract, forming ionic bonds, which create the vast diversity of ionic compounds.
Mastering Memorization
Flashcards, mnemonics, and repetition are effective memorization techniques. Experiment to find the best approach for you.
Mastering Polyatomic Ions: Quiz for Beginners
Ready to test your knowledge? This quiz focuses on identifying common polyatomic ions.
(Quiz questions and answers would be placed here)
Unlocking the Secrets of Naming
The “-ate” and “-ite” suffixes are key to naming conventions. “-ate” generally denotes more oxygen atoms than “-ite.” Prefixes like “per-” and “hypo-” further refine the system. Understanding these patterns simplifies memorization.
Memorization Techniques
Don’t rely on brute force memorization. Flashcards, mnemonics, and writing practice are effective strategies. Experiment to discover what suits you.
Real-World Applications
Polyatomic ions are found in everyday items, like baking soda and fertilizers, and play critical roles in biological processes.
This comprehensive guide equips you with the knowledge and resources to master polyatomic ions, from basic definitions to advanced concepts and practical applications. Remember, consistent practice and strategic learning are key to success.
- Revolution Space: Disruptive Ion Propulsion Transforming Satellites - April 24, 2025
- Race Through Space: Fun Family Game for Kids - April 24, 2025
- Unlocking the Universe: reading about stars 6th grade Guide - April 24, 2025