Hey there, future MCAT achievers! The periodic table is your ally on test day, not your enemy. Forget memorizing every detail—you’ll have a periodic table provided. This guide reveals how to decode its secrets, leverage its power, and boost your MCAT chemistry score.
Deciphering Periodic Trends for MCAT Success
The MCAT provides a periodic table, a valuable tool often overlooked. It’s not about memorization, but strategy. This section explores how to wield this tool effectively.
Unlocking the Secrets: Periodic Trends
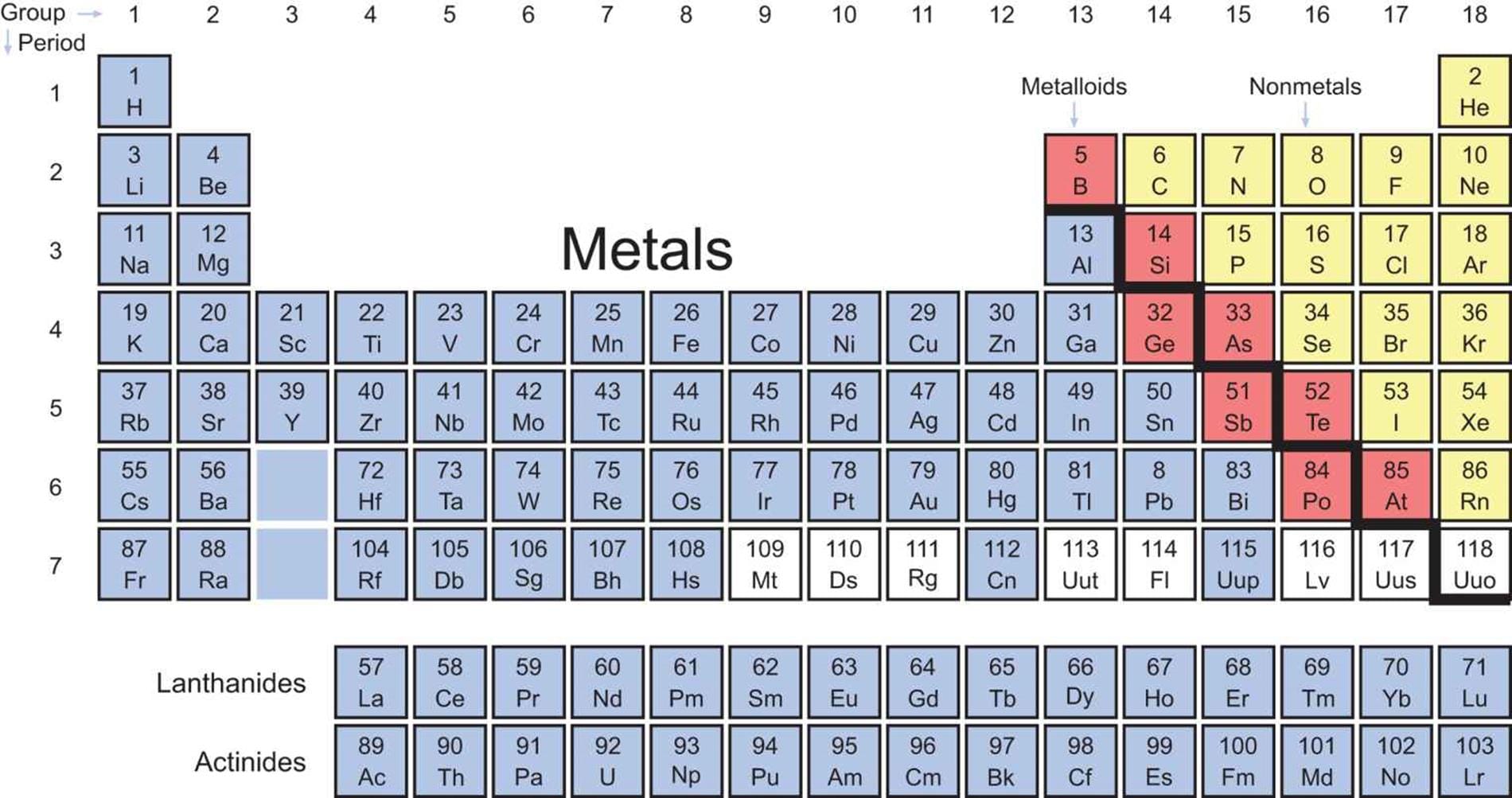
Visualize the periodic table as a map, not just a list. It’s organized by periodic trends, predictable patterns in element properties. These trends, like electronegativity (an atom’s electron attraction), ionization energy (energy to remove an electron), and atomic radius (an atom’s size), are crucial for predicting element behavior. [https://www.lolaapp.com/] For instance, electronegativity generally increases across a period and decreases down a group. Thus, fluorine, in the top right, is highly electronegative, while francium, bottom left, is far less so. Mastering these trends enables educated predictions about unfamiliar elements.
Practice Makes Perfect: Applying Periodic Trends
Familiarizing yourself with the MCAT periodic table involves active application, not passive observation. Practice questions that rely on the table strengthen problem-solving skills and highlight necessary information extraction. The more you practice, the more intuitive it becomes. [https://www.lolaapp.com/]
Know Your Territory: The MCAT Periodic Table
The MCAT periodic table is streamlined. It contains essential information but not the comprehensive detail of a textbook version. Familiarize yourself with its specific content and presentation. Knowing what’s included (and what’s not) is key. Does it have electron configurations? Are common isotopes listed? [https://www.lolaapp.com/]
Time is of the Essence: Efficient Table Use
The MCAT is timed, so efficient information retrieval is critical. Practice smooth navigation if it’s digital. If it’s a paper version, locate information rapidly without frantic searching. Every second counts. [https://www.lolaapp.com/]
Confidence is Key: Approach the Table Strategically
The periodic table is a tool, not a test itself. Focus on understanding trends, practicing problem-solving, and efficient table use. This transforms a potential anxiety source into a powerful ally.
Is a Periodic Table Provided on the MCAT?
Yes, a periodic table is provided on the MCAT, a significant advantage. However, it’s a simplified version, including element symbols, atomic numbers, and atomic masses. Don’t expect detailed information like electronegativity values or electron configurations. This underscores the importance of understanding periodic trends to extrapolate information. [https://www.lolaapp.com/] While direct questions on specific properties are unlikely, understanding trends is crucial, especially for organic chemistry, where predicting reactions and molecular behavior hinges on valency and hybridization. Strategic studying, focusing on trends and their application, is key. [https://www.lolaapp.com/]
Focusing Your MCAT Periodic Table Prep
Here’s a breakdown of key periodic table concepts for the MCAT:
| Concept | Importance | Example |
|---|---|---|
| Electronegativity | Predicts bond polarity and reactivity | Comparing the reactivity of fluorine and chlorine |
| Ionization Energy | Relates to how easily an atom loses an electron | Understanding why alkali metals are so reactive |
| Atomic Radius | Influences bonding and intermolecular forces | Explaining the difference in boiling points between similar molecules |
| Valence Electrons | Determines bonding capacity and molecular geometry | Predicting the shape of a molecule |
| Hybridization | Crucial for understanding bonding in organic molecules | Explaining the difference between single, double, and triple bonds |
| Periodic Trends | Provides a framework for understanding element behavior | Predicting the acidity or basicity of a compound |
| Ionic States | Important for predicting reaction products and balancing chemical equations | Knowing the common charges of different elements |
Ongoing research continually refines our understanding of periodic properties. While the MCAT focuses on established principles, our knowledge evolves. The provided periodic table is a tool; your understanding maximizes its potential.
Memorizing the Periodic Table for the MCAT: Necessary or Not?
The MCAT provides a periodic table, so complete memorization is unnecessary. However, understanding the why behind the arrangement is crucial. Focus on the “landmarks”—periodic trends—like electronegativity, ionization energy, and atomic radius. These trends predict element properties without memorizing every detail. The MCAT assesses your ability to apply these concepts, not simply recall facts. [https://www.lolaapp.com/] For instance, comparing element reactivity relies on understanding electronegativity trends.
Effectively Using the Periodic Table on the MCAT
Grasp the Big Picture: Understand periods, groups, and their relationship to electron configuration and properties.
Master the Trends: Focus on electronegativity, ionization energy, atomic radius, and electron affinity.
Practice, Practice, Practice: Actively use the periodic table during practice problems. [https://www.lolaapp.com/]
Familiarize Yourself with the MCAT Version: Ensure you’re comfortable with the digital or paper version provided.
Supplement Your Learning: Use flashcards and online quizzes to reinforce trend comprehension.
Memorizing vs. Understanding
| Feature | Memorizing the Entire Table | Understanding Periodic Trends |
|---|---|---|
| Time Commitment | High | Moderate |
| MCAT Relevance | Low | High |
| Long-Term Benefit | Limited | Enables problem-solving |
| Practicality | Difficult | More efficient |
Understanding trends is more efficient and applicable than rote memorization. Ongoing research continually refines our understanding. Focus on trends for MCAT success. This enables educated predictions about element behavior based on their position. [https://www.lolaapp.com/]
How the Periodic Table is Used in MCAT Chemistry
The periodic table’s power lies in its ability to predict element behavior. The MCAT assesses your ability to use it as a tool, not just memorize it. Can you predict reactivity or bond strength based on its structure? Understanding periodic trends is essential.
Key Periodic Trends
Electronegativity: An atom’s “electron hunger,” influencing bonding.
Atomic Radius: An atom’s size, impacting interactions with other atoms.
Ionization Energy: Energy required to remove an electron.
Electron Affinity: Energy released when an atom gains an electron.
Navigating the Periodic Table
Know the Trends: Electronegativity generally increases across a period and decreases down a group. Atomic radius follows the opposite pattern. Ionization energy and electron affinity generally follow electronegativity.
Apply Your Knowledge: Use trends to deduce properties, predict reactions, and understand bonding.
Practice Makes Perfect: Practice applying these concepts through MCAT practice problems.
MCAT Periodic Table Tips
Relax: A periodic table is provided.
Focus on Trends: Understand the general patterns, not specific values.
Apply, Apply, Apply: Practice using it to solve problems.
Strategize: Develop and follow a study plan.
| Trend | Across a Row | Down a Column |
|---|---|---|
| Electronegativity | Increases | Decreases |
| Atomic Radius | Decreases | Increases |
| Ionization Energy | Increases | Decreases |
| Electron Affinity | Increases | Decreases |
Ongoing research refines our understanding of periodic trends. This knowledge is constantly evolving. [https://www.lolaapp.com/realidades-2-workbook-answers] [https://www.lolaapp.com/realidades-2-workbook-answers-pdf]
Recommended Titles:
Mastering the MCAT Periodic Table: Trends, Strategies, and Practice Questions
Decoding the MCAT Periodic Table: Your Test-Day Companion
Beyond Memorization: Conquering MCAT Chemistry with Periodic Table Mastery
Powerful Key Lines:
Unlock MCAT Success: Master periodic trends, not just elements.
Simplified MCAT Chemistry: Use the provided table as your guide.
MCAT Periodic Table Advantage: Maximize your test-day performance.
Ace MCAT Chemistry: Turn the periodic table into your secret weapon.
Important Details:
Availability: Provided during the exam.
Content: Element symbol, atomic number, and atomic mass.
Importance of Trends: Crucial for problem-solving.
Access: Digitally on-screen.
Practice: Essential for developing skills.
Structured Context:
I. MCAT Provides a Periodic Table: Memorization is unnecessary; focus on application.
II. Content: Basic information only; supplemental knowledge of trends is key.
III. How to Use: Master trends and practice problem-solving.
IV. Resources: AAMC resources, practice questions, test prep companies.
Unique Insights & Untapped Potential:
In-depth problem-solving: Go beyond simply mentioning “don’t memorize.”
Bridging the gap: Connect basic knowledge with advanced concepts.
Addressing test anxiety: Offer confidence-building strategies.
Interactive elements: Enhance engagement with quizzes and practice questions (consider adding these in the future).
- Crypto Quotes’ Red Flags: Avoid Costly Mistakes - June 30, 2025
- Unlock Inspirational Crypto Quotes: Future Predictions - June 30, 2025
- Famous Bitcoin Quotes: A Deep Dive into Crypto’s History - June 30, 2025



![Here are a few title options that incorporate your keywords, reflect current trends, and mirror competitor approaches:Direct & Actionable:AP Calculus BC Score Calculator (2023-2024): Predict & Improve Your Score
Free AP Calculus BC Score Calculator: 2024 Predictions & Exam GuideBenefit-Driven:Get a 5 on the AP Calculus BC Exam: Score Calculator & Study Guide
Ace Your AP Calculus BC Exam: Score Predictor & Prep ResourcesAdding Specificity (if applicable):[Your Website's Name] AP Calculus BC Score Calculator: 2024 ExamTips for Choosing the Best Title:Analyze Competitor Titles: Look at the top-ranking pages for AP Calc BC score calculator. Identify common themes and phrases.
Target Long-Tail Keywords: Consider longer, more specific phrases users might search, like how accurate are AP Calculus BC score calculators.
A/B Testing: If possible, test different titles to see which performs best in terms of clicks and engagement. ap_calc_bc_score_calculator](https://www.lolaapp.com/wp-content/uploads/2024/10/ap_calc_bc_score_calculator-150x150.jpg)










