This guide reveals the secrets to maximizing your MCAT score by strategically using the provided periodic table. We’ll explore essential trends, offer practice strategies, and delve into how the periodic table unlocks key chemistry concepts.
Decoding the MCAT’s Periodic Table
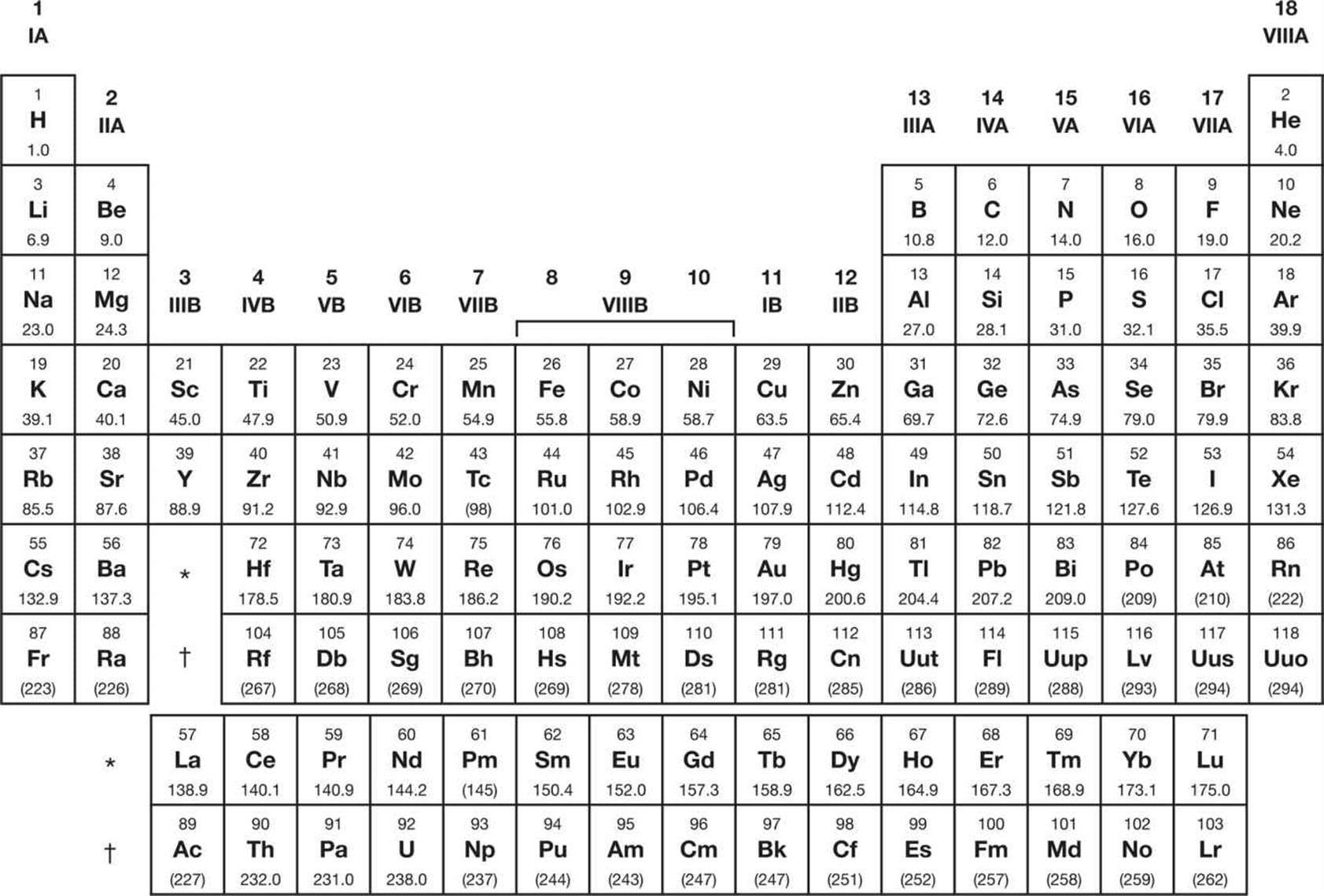
The MCAT provides a streamlined periodic table—your roadmap for navigating the complexities of MCAT chemistry. This version, while simplified, contains crucial information: atomic numbers, atomic masses, and element symbols. It’s essential to understand how this tool can empower you to solve problems and reason through chemical scenarios. For additional study resources, consider exploring our Little Seagull Handbook for language arts and MasteryConnect Code for coding skills.
Understanding Periodic Trends
A key to utilizing the MCAT periodic table effectively is understanding periodic trends. These trends predict how element properties change across and down the table. A helpful mnemonic is BEAR:
- Basicity: Increases up and to the left.
- Electronegativity, Electron Affinity, and Ionization Energy: Generally increase up and to the right.
- Radius: Increases down and to the left.
Memorizing BEAR isn’t enough. Understanding why these trends exist is crucial. For instance, electronegativity increases up and to the right likely because the nucleus’ positive charge exerts a stronger pull on electrons in smaller atoms.
Is a Periodic Table Given on the MCAT?
Yes, a simplified periodic table is provided on the MCAT. It contains the basics (element symbols, atomic numbers, and atomic masses), but lacks details like electronegativity values and electron configurations. This streamlined version encourages a focus on broader concepts and periodic trends, essential for predicting how elements interact. Some experts believe this focus on trends fosters a deeper understanding of chemical behavior than memorizing specific values. Ongoing research continues to explore the nuances of periodic trends, suggesting that our current understanding may evolve. However, this shouldn’t discourage you. Instead, embrace the simplified table as an opportunity to demonstrate your understanding of trends and problem-solving skills.
How the Periodic Table Unlocks MCAT Chemistry
The MCAT periodic table is vital for solving chemistry problems. It helps determine the elements in a reaction, predict reaction outcomes, understand compound properties, and grasp relationships between elements. For instance, knowing sodium (Group 1) has one valence electron and chlorine (Group 17) has seven, suggests they will probably combine to form sodium chloride in a 1:1 ratio. The table also reveals that sodium is a metal and chlorine a nonmetal, indicating that sodium chloride is likely a solid at room temperature and may have a high melting point. While our understanding of the periodic table is well-established, ongoing research continues to refine our knowledge.
| What you want to know | How the periodic table helps |
|---|---|
| Elements involved in a reaction | Identifies elements by symbol and group/period placement |
| Products of a reaction | Predicts product formation based on valence electron configuration |
| Properties of a compound | Provides insight into properties based on the elements involved |
| Relationships between different elements | Shows trends and similarities based on group and period placement |
What’s on the MCAT Periodic Table?
The MCAT, a critical exam for aspiring doctors, assesses a wide range of scientific knowledge, with chemistry being a core component. Within chemistry, the periodic table is a pivotal tool. The MCAT provides a streamlined version containing element symbols, atomic numbers, and atomic masses. This simplified format encourages a focus on understanding periodic trends—electronegativity, atomic radius, ionization energy, and electron affinity—which are vital for predicting element behavior in chemical reactions.
The MCAT periodic table strategically omits electronegativity values and electron configurations. This design pushes test-takers to apply their understanding of periodic trends rather than relying on rote memorization. Mastering these trends unlocks a deeper understanding of chemistry.
| Feature | Included on MCAT Periodic Table? |
|---|---|
| Element Symbols | Yes |
| Atomic Numbers | Yes |
| Atomic Masses | Yes |
| Electronegativity | No |
| Electron Config. | No |
While current scientific understanding suggests these trends are generally reliable, ongoing research may further refine these concepts.
Key Takeaways
- Strategic Use is Key: The MCAT periodic table is a tool for problem-solving, not just a chart of elements.
- Trends Over Memorization: Focus on understanding periodic trends, not memorizing the entire table.
- Practice Makes Perfect: Utilize practice materials from the AAMC and third-party resources to hone your skills.
- Simplified Table, Deeper Understanding: The streamlined table encourages a deeper understanding of underlying chemical principles.
- Continuous Learning: Our understanding of chemistry and periodic trends is constantly evolving with ongoing research.
By mastering the MCAT periodic table and its underlying principles, you’ll be well-equipped to conquer the MCAT chemistry section. Remember, it’s not about rote memorization; it’s about understanding the “why” behind the “what.”
- Unlock Water’s Symbolism: A Cross-Cultural Exploration - April 20, 2025
- Identify Black and White Snakes: Venomous or Harmless? - April 20, 2025
- Unlocking Potential: Origins High School’s NYC Story - April 20, 2025


![Here are a few title options that incorporate your keywords, reflect current trends, and mirror competitor approaches:Direct & Actionable:AP Calculus BC Score Calculator (2023-2024): Predict & Improve Your Score
Free AP Calculus BC Score Calculator: 2024 Predictions & Exam GuideBenefit-Driven:Get a 5 on the AP Calculus BC Exam: Score Calculator & Study Guide
Ace Your AP Calculus BC Exam: Score Predictor & Prep ResourcesAdding Specificity (if applicable):[Your Website's Name] AP Calculus BC Score Calculator: 2024 ExamTips for Choosing the Best Title:Analyze Competitor Titles: Look at the top-ranking pages for AP Calc BC score calculator. Identify common themes and phrases.
Target Long-Tail Keywords: Consider longer, more specific phrases users might search, like how accurate are AP Calculus BC score calculators.
A/B Testing: If possible, test different titles to see which performs best in terms of clicks and engagement. ap_calc_bc_score_calculator](https://www.lolaapp.com/wp-content/uploads/2024/10/ap_calc_bc_score_calculator-150x150.jpg)











2 thoughts on “Conquering the MCAT Periodic Table: Trends, Strategies, and Practice”
Comments are closed.