Hydrogen cyanide (HCN) is a simple yet fascinating, and potentially dangerous, molecule. Understanding its structure is key to understanding its properties. This guide provides a step-by-step approach to constructing the HCN Lewis dot structure, deciphering its molecular geometry, and understanding its polarity. Along the way, we’ll explore the implications of this structure and its relevance in various scientific fields.
Deciphering the HCN Lewis Dot Structure
Lewis dot structures are essential tools in chemistry, providing a visual representation of valence electrons and bonding within a molecule. They serve as blueprints, revealing how atoms connect and how these connections influence the molecule’s overall behavior. Let’s delve into the process of constructing the Lewis dot structure for hydrogen cyanide (HCN).
Building the HCN Lewis Dot Structure: A Step-by-Step Guide
Count Valence Electrons: Begin by determining the total number of valence electrons available.
- Hydrogen (H) contributes 1 valence electron.
- Carbon (C) contributes 4 valence electrons.
- Nitrogen (N) contributes 5 valence electrons.
- Total: 1 + 4 + 5 = 10 valence electrons
Identify the Central Atom: The least electronegative atom (excluding hydrogen) typically occupies the central position. In HCN, carbon (C) is the central atom.
Connect Atoms with Single Bonds: Draw single bonds between the central atom (C) and the surrounding atoms (H and N). This uses 2 x 2 = 4 electrons, so we have 6 remaining.
Satisfy the Octet Rule: Atoms generally strive to achieve a full outer shell of eight electrons (the octet rule), with the exception of hydrogen, which aims for a duet (2 electrons). To satisfy the octet rule for both carbon and nitrogen in HCN, we need to introduce multiple bonds. A triple bond forms between carbon (C) and nitrogen (N), utilizing the remaining 6 valence electrons.
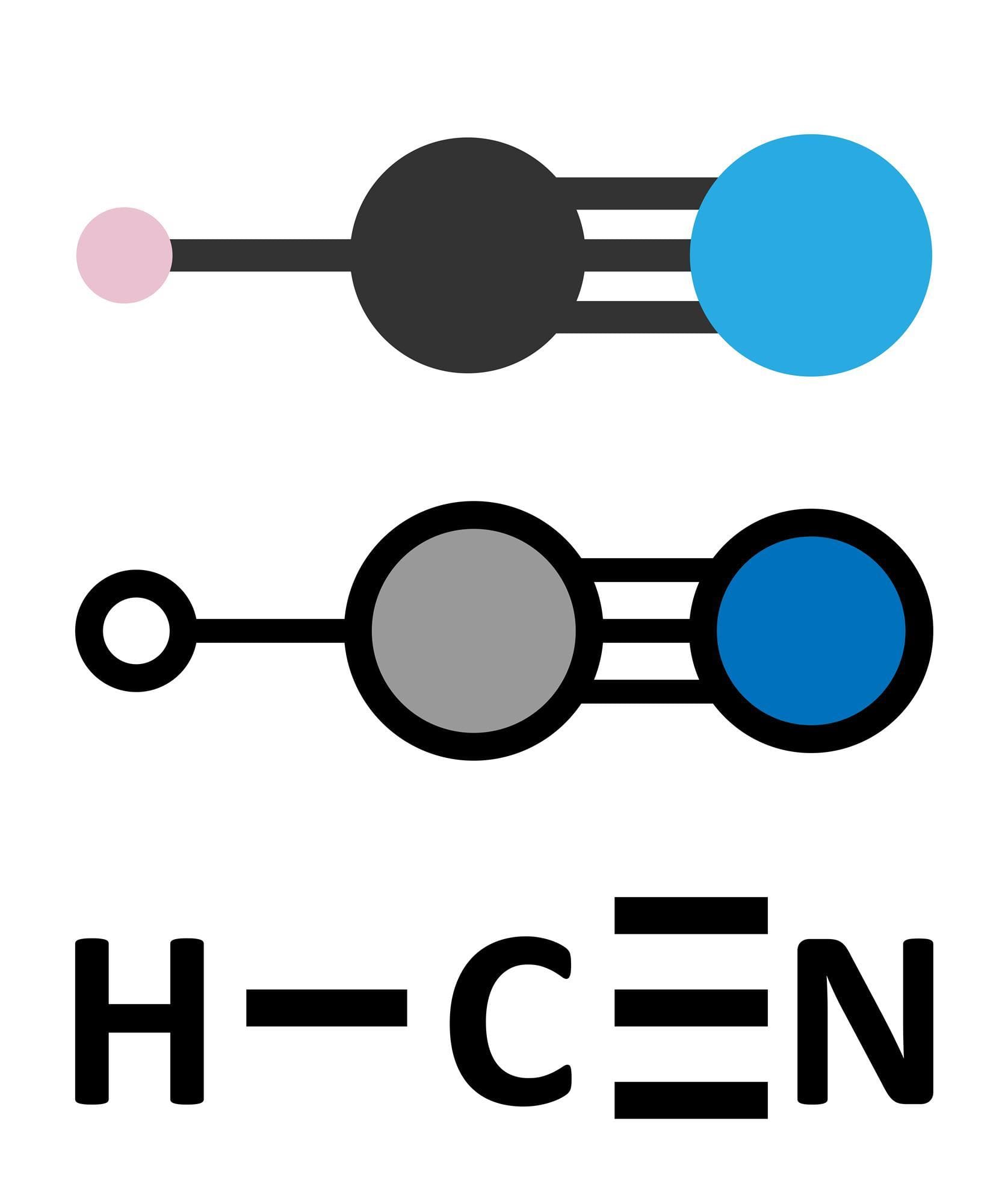
Final Lewis Structure: The final Lewis structure for HCN is represented as: H–C≡N: Nitrogen has a lone pair of electrons completing the octect. A diagram of this is shown below:
..
H - C ≡ N:
[Diagram of Lewis Dot Structure for HCN would go here]
Understanding HCN’s Geometry and Polarity
The Lewis structure unlocks crucial insights into HCN’s shape and charge distribution.
Molecular Geometry: Linear Structure
The arrangement of electron pairs around the central carbon atom dictates HCN’s linear molecular geometry. The carbon atom has two electron regions – one single bond and one triple bond. These regions repel each other, resulting in a straight line arrangement (180° bond angle) of the hydrogen, carbon, and nitrogen atoms.
Molecular Polarity: Uneven Charge Distribution
HCN is a polar molecule due to the significant electronegativity difference between carbon and nitrogen. Nitrogen, being more electronegative, attracts the shared electrons in the triple bond more strongly. This creates a partial negative charge (δ-) on the nitrogen atom and a partial positive charge (δ+) on the carbon and hydrogen atoms. This uneven charge distribution results in a dipole moment, making the molecule polar.
HCN: Real-World Implications and Ongoing Research
Understanding the Lewis dot structure of HCN is not merely an academic exercise; it has practical implications across various fields.
Applications and Significance
- Industrial Chemistry: HCN serves as a crucial precursor in the synthesis of various polymers and other industrial chemicals.
- Agriculture: It’s used in fumigants for pest control.
- Cigarette Smoke: HCN is a component of cigarette smoke, contributing to its toxicity.
- Astrochemistry: HCN has been detected in interstellar space, prompting further research into its role in the formation of stars and planets.
Ongoing Research and Nuances
While the standard Lewis dot structure is a valuable tool, it’s important to acknowledge its limitations. Ongoing research delves into more complex models, like molecular orbital theory, to provide a more nuanced understanding of HCN’s electron distribution and bonding. Additionally, the study of HCN’s reactivity, particularly regarding the triple bond’s susceptibility to addition reactions, remains an active area of investigation. Some researchers are exploring how HCN interacts with other molecules in different environments, furthering our understanding of this important and complex molecule.
Are you interested in learning about the lewis structure of sf3? Our experts have created a page to take a deep dive into this topic and provide you with all the information you need.