Embark on a journey into the world of molecular chemistry as we unravel the structure of hydrogen cyanide (HCN) through its Lewis structure. This guide provides a detailed exploration of HCN’s molecular architecture, offering insights into its geometry, polarity, and chemical bonds. Discover the secrets within the HCN Lewis structure – a key to understanding this remarkable molecule.
Decoding the HCN Lewis Structure
Lewis structures provide a glimpse into the architecture of molecules. They illustrate how atoms connect and where electrons reside. This guide offers a step-by-step approach to drawing the HCN Lewis structure, using accessible language for all levels of chemistry knowledge.
Why Lewis Structures Matter
Understanding a Lewis structure is fundamental to predicting a molecule’s behavior – its reactivity, shape, and physical properties. It’s the foundation for comprehending how molecules interact.
Getting Started
- Valence Electrons: These outermost electrons participate in bonding.
- Periodic Table: Use it to determine the number of valence electrons for each atom.
Drawing the HCN Lewis Structure: A Step-by-Step Guide
-
Count Valence Electrons: Hydrogen (H) has 1, carbon (C) has 4, and nitrogen (N) has 5. Total: 1 + 4 + 5 = 10 valence electrons.
-
Connect the Atoms: Carbon is the central atom. Connect hydrogen to carbon and carbon to nitrogen with single bonds, representing two shared electrons each. Four electrons used.
-
Distribute Remaining Electrons: Six electrons remain. Place these around the nitrogen atom. Nitrogen likely aims for an octet (8 electrons).
-
Form the Triple Bond: Nitrogen needs two more electrons for its octet. Form a triple bond between carbon and nitrogen using the remaining six electrons (two for each bond).
-
Verify Octets/Duets: Hydrogen has a duet (2 electrons). Carbon and nitrogen each have an octet.
| Atom | Valence Electrons | Electrons in HCN |
|---|---|---|
| H | 1 | 2 (shared) |
| C | 4 | 8 (shared) |
| N | 5 | 8 (6 shared, 2 lone pair) |
Unveiling HCN’s Molecular Shape
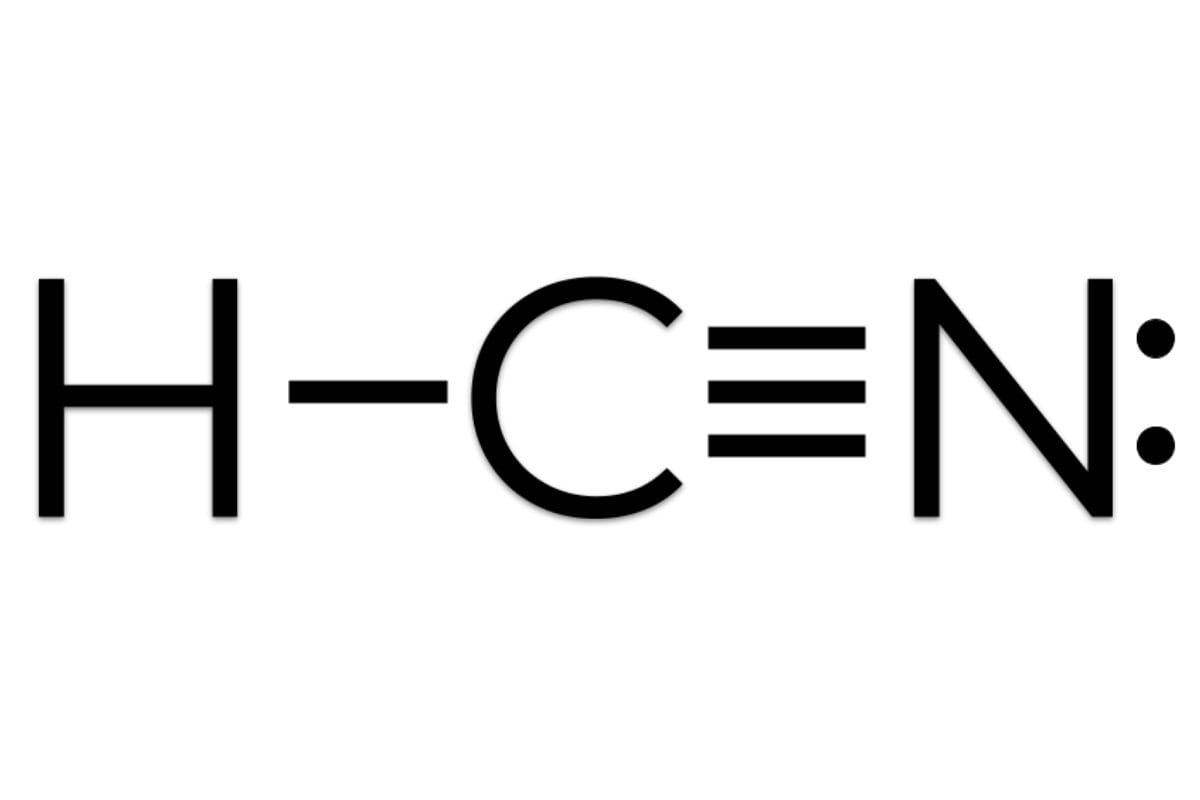
HCN is a linear molecule (H-C≡N) due to the strong triple bond between carbon and nitrogen. The triple bond (≡) represents a strong connection involving six shared electrons. The hydrogen atom bonds with carbon through a single bond.
This linear structure significantly impacts HCN’s properties and interactions with other molecules, influencing its reactivity and behavior in chemical reactions. It’s this linear arrangement that contributes to HCN’s distinct characteristics.
Exploring the Bonds Within HCN
HCN exhibits a fascinating interplay of different bond types. The hydrogen and carbon atoms are connected by a single sigma (σ) bond – a direct, head-on overlap of atomic orbitals.
The carbon and nitrogen atoms, however, are joined by a triple bond, consisting of one sigma (σ) bond and two pi (π) bonds. Pi bonds arise from the sideways overlap of atomic orbitals, creating a stronger and shorter bond than the single sigma bond. This triple bond significantly contributes to HCN’s linear geometry and overall stability.
Deciphering HCN’s Polarity
Nitrogen’s higher electronegativity compared to carbon results in an uneven charge distribution, making HCN a polar molecule. This polarity influences its interactions with other polar molecules and contributes to its solubility in polar solvents like water.
HCN in the Real World
HCN is used in producing certain plastics and is found in some fruit pits (in small, generally harmless amounts). It’s also highly toxic. Understanding its structure helps explain its reactivity.
Ongoing Research and Future Directions
Research continues to explore the nuances of HCN’s bonding and behavior. Scientists are investigating its role in various chemical reactions and its potential applications in different fields, including its possible role in the origin of life.
Conclusion
The HCN Lewis structure, a simplified representation, provides insights into this molecule’s characteristics. Did you know that the H-N-O Lewis structure also offers a visual representation of electron arrangement? The HCN Lewis structure is a testament to the power of these structures to unveil the secrets of the molecular world. It’s a foundation for understanding more complex theories and models.
HCN: A Summary of Key Properties
| Feature | Description |
|---|---|
| Formula | HCN |
| Structure | Linear (H-C≡N) |
| Valence Electrons | 10 |
| Bond Types | Single bond (H-C, σ), Triple bond (C≡N, 1 σ, 2 π) |
| Polarity | Polar (due to electronegativity difference between C and N) |
| Geometry | Linear (180° bond angle) |
HCN’s linear structure and polarity greatly influence its interactions and reactivity. Ongoing research continues to uncover the full extent of HCN’s chemical behavior in diverse environments.
Further Exploration of HCN’s Geometry
HCN’s linear geometry is a direct consequence of the electron arrangement and bonding. The strong triple bond between carbon and nitrogen, combined with the single bond to hydrogen, dictates the 180° bond angle, classifying it as an AX2 molecule according to VSEPR theory. While molecular vibrations occur, the average shape remains linear.
The Significance of HCN’s Bonding
The combination of sigma and pi bonds in HCN creates a unique electronic environment. The triple bond between carbon and nitrogen is significantly stronger and shorter than the H-C single bond, influencing HCN’s reactivity and stability. This complex bonding arrangement contributes to HCN’s unique chemical personality.
Key Points to Remember:
- HCN has single (H-C) and triple (C≡N) bonds.
- The C≡N triple bond includes one sigma (σ) and two pi (π) bonds.
- HCN is linear with a 180° bond angle.
- HCN is polar due to the electronegativity difference between C and N.
This comprehensive guide provides a solid foundation for understanding the HCN Lewis structure and its implications. Further exploration into advanced molecular modeling can offer even deeper insights into this fascinating molecule.
- Unveiling Bernhard Caesar Einstein’s Scientific Achievements: A Legacy in Engineering - July 15, 2025
- Uncover who is Jerry McSorley: CEO, Family Man, Business Success Story - July 15, 2025
- Discover Bernhard Caesar Einstein’s Scientific Contributions: Unveiling a Legacy Beyond Einstein - July 15, 2025














1 thought on “Understanding the HCN Lewis Structure: A Comprehensive Guide”
Comments are closed.