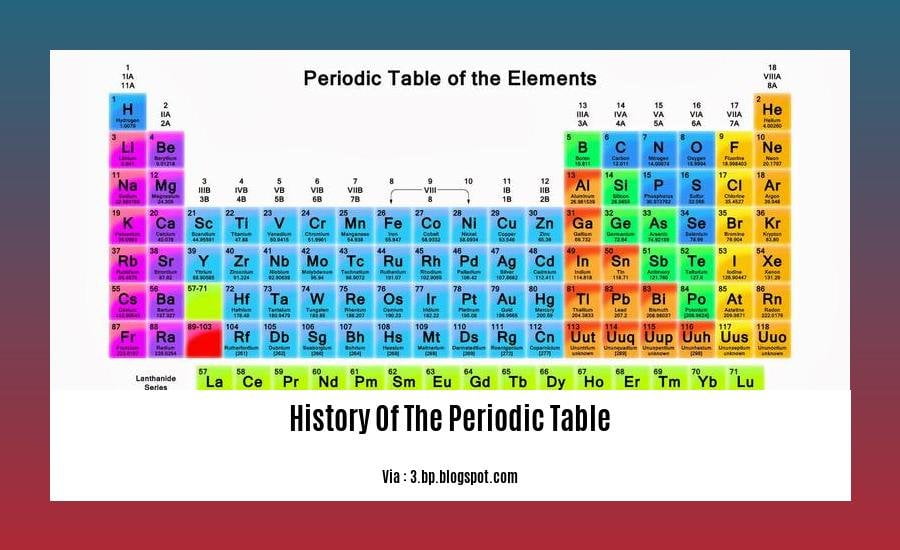
Embark on a scientific odyssey with [The Evolution of Order: A Journey Through the History of the Periodic Table]. Dive into the captivating tale of the periodic table, conceived by Dmitri Mendeleyev in the 1860s. Far from being the first, this table laid the groundwork for a standardized understanding of the elements. From the discovery of man-made elements to the pivotal role of increasing atomic numbers in shaping its structure, join us as we unravel the remarkable evolution of this cornerstone of chemistry.
Key Takeaways:
- Ancient Element Knowledge: Nine elements known before antiquity.
- Aristotle’s Theory: All matter made from “roots” (elements).
- Periodic Element Properties: Properties vary periodically with atomic weight.
- 19th Century Pioneers: Mendeleev, Newlands, and Meyer created early periodic tables.
- 20th Century Advancements: Discovery of radioactivity, isotopes, and new elements.
History of the Periodic Table

Unfolding the history of the periodic table is a captivating journey through the evolution of science. The periodic table, the iconic chart organizing chemical elements, is a cornerstone of chemistry and a testament to the scientific endeavor.
Early Insights
Before the history of the periodic table began, humans recognized a handful of elements, like gold and iron. Ancient philosophers like Aristotle theorized that all matter comprised a mix of root elements.
Dawn of Periodicity
The concept of periodicity emerged in the 19th century. Scientists observed a pattern: element properties repeated at regular intervals based on atomic weight.
Mendeleev’s Masterpiece
In 1869, Dmitri Mendeleev published his periodic table, a groundbreaking work that arranged elements by atomic weight and properties. Mendeleev even predicted the existence of undiscovered elements based on gaps in his table.
Ongoing Evolution
The history of the periodic table continued with discoveries of radioactivity and isotopes. New elements were identified, expanding the table and confirming Mendeleev’s foresight. Today, the periodic table remains a dynamic tool, constantly updated with new knowledge and insights.
Discover the remarkable contributions of Dmitri Mendeleevs contributions to chemistry, including his groundbreaking work on the discovery of elements and the development of the modern table organization.
3. Out of One Hundred and Eighteen Elements, 20 are Man-Made!
Key Takeaways:
- The periodic table has 118 elements, including 20 man-made elements.
- The first artificial element was technetium.
- Man-made elements are essential in fields such as medicine, technology, and energy.
The Birth of Synthetic Elements
From the primordial soup of the early universe to the complex tapestry of matter we witness today, the journey of elements has been an epic tale. Among the 118 elements that grace the periodic table, 20 stand out as a testament to human ingenuity—these are the man-made elements.
Technetium: The First-Born
In 1937, technetium emerged as the first artificial element, birthed in the crucible of nuclear reactions. This milestone marked the dawn of an era where scientists could transcend the confines of nature’s elemental palette.
A Symphony of Creation
The creation of man-made elements is a delicate dance of nuclear physics. By bombarding stable elements with high-energy particles, scientists can coax them into forming new, unstable isotopes. These isotopes may decay rapidly, but they offer invaluable insights into the fundamental forces that govern matter.
Harnessing the Power of Atoms
Man-made elements are not merely scientific curiosities; they play a vital role in modern society. They find applications in:
- Medicine: Radioisotopes for medical imaging and therapy (e.g., iodine-131 for thyroid treatment)
- Technology: Gadolinium for MRI contrast agents, neodymium for lasers
- Energy: Plutonium and uranium for nuclear power, americium for smoke detectors
A Testament to Human Curiosity
Each man-made element is a testament to the relentless pursuit of knowledge and the indomitable human spirit. As we continue to unravel the secrets of the periodic table, we push the boundaries of scientific understanding and pave the path for transformative technologies that will shape the future of our world.
Citation:
- MyTutorSource:
11. The Order of the Increasing Atomic Numbers was Used to Design the Periodic Table
A key breakthrough in the evolution of the periodic table occurred when scientists like J.A.R. Newlands proposed organizing elements based on their increasing atomic weights. This was a crucial step because it allowed for the identification of patterns and similarities among elements.
Assigning elements ordinal numbers and grouping them into categories based on their resemblance to known elements like hydrogen, lithium, and others provided a framework for understanding the chemical properties of elements.
Key Takeaways:
- Organizing elements based on increasing atomic weights revealed patterns and similarities among them.
- Assigning ordinal numbers and grouping elements into categories based on their resemblances provided a framework for understanding their chemical properties.
- This discovery laid the groundwork for the development of the modern periodic table.
Relevant URL Sources:
FAQ
Q1: Who presented the periodic table in the 1860s?
A1: Dmitri Mendeleev is credited with presenting the periodic table in the 1860s.
Q2: Was this periodic table the first standard periodic table?
A2: No, the periodic table presented by Mendeleyev was not the first standard periodic table.
Q3: How many of the 118 elements are man-made?
A3: Out of 118 elements, 20 are man-made.
Q4: What was the basis for designing the periodic table?
A4: The order of the increasing atomic numbers was used to design the periodic table.
Q5: What are the rows of the periodic table called?
A5: Periods
- China II Review: Delicious Food & Speedy Service - April 17, 2025
- Understand Virginia’s Flag: History & Debate - April 17, 2025
- Explore Long Island’s Map: Unique Regions & Insights - April 17, 2025