Ever wondered if liquids are as well-behaved as they seem? Do they always take up the exact same amount of space no matter what? Let’s dive into the world of liquids and explore whether they have a mind of their own when it comes to volume. Join us on a scientific adventure as we uncover the secrets of these enigmatic fluids through a physicist’s lens.
Does Liquid Have a Definite Volume?
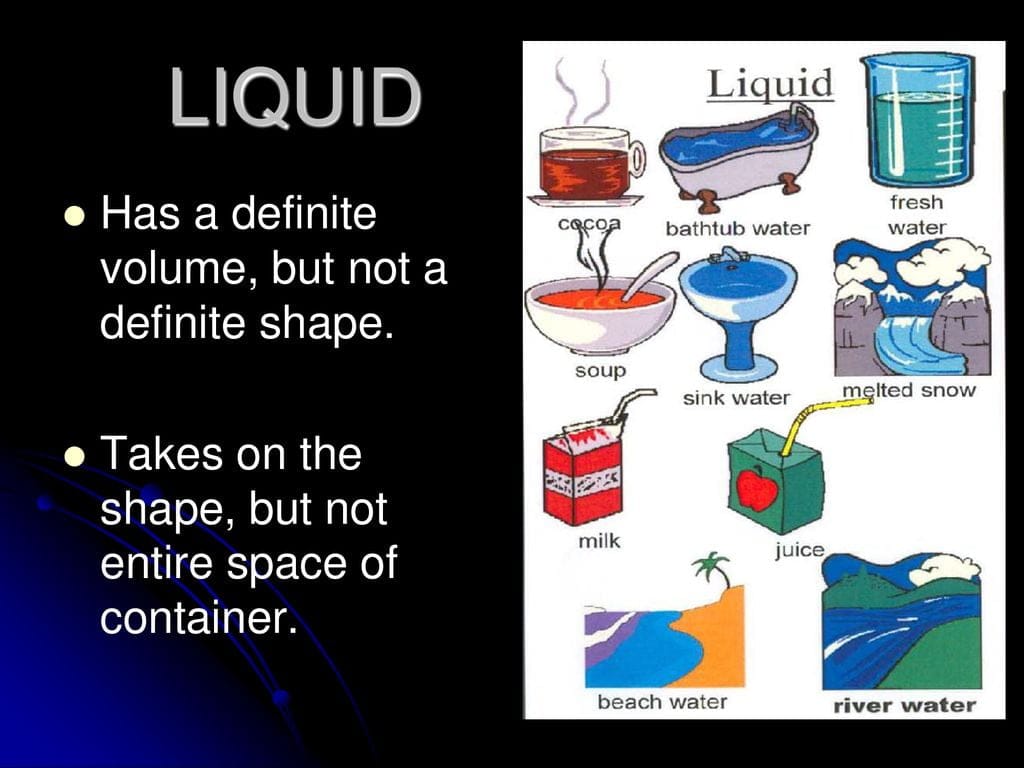
You know how juice, when poured into a glass, takes the shape of the glass but doesn’t continue spreading out like air? That’s because liquids do have a definite volume. They exist in a state of matter that’s not too rigid like solids, but also not as free-flowing as gases.
Think about it: a liter of milk will always be a liter of milk, whether it’s in a carton, a glass, or (hopefully not) splashed on the floor. This is because the molecules in a liquid, while more mobile than those in a solid, are still much closer together than those in a gas. This means a liter of liquid will always occupy roughly the same amount of space.
It’s important to note that this volume is not perfectly fixed. Heating a liquid can cause its molecules to become more energetic and spread out slightly, leading to a slight expansion in volume. Conversely, extreme pressure can force the molecules closer together, resulting in a small decrease in volume. However, under most everyday circumstances, these changes are negligible.
This relatively constant volume of liquids is crucial for numerous applications. Imagine trying to design a hydraulic system, like the brakes in your car, using a substance whose volume changes constantly–it simply wouldn’t work! Our ability to reliably measure and manipulate liquids based on their consistent volume is essential for countless technologies and everyday processes.
While scientists continue to unravel the intricacies of liquid behavior, particularly under extreme conditions, the next time you pour yourself a glass of something refreshing, take a moment to appreciate the wonder of liquids and their “just-right” volume!
Does Gas Have a Definite Volume?
We’ve touched upon how solids and liquids occupy space, but what about gases? They seem to behave quite differently. Think about blowing up a balloon. The air you blow in, a gas, doesn’t simply pool at the bottom. Instead, it expands to fill the entire balloon, altering both its shape and size.
This difference in behavior boils down to the interactions between the tiny particles that constitute matter in different states. In solids, these particles are tightly packed, with minimal freedom of movement. This results in a fixed shape and volume. In liquids, the particles have a bit more freedom to move around but are still relatively close together, giving them a definite volume but not a fixed shape.
However, gas particles are like tiny, energized balls bouncing around at high speeds. They are much farther apart than liquid or solid particles and interact much less with each other. This constant, chaotic movement means a gas doesn’t have a fixed shape or volume. It will continue to expand to fill whatever container it’s in, much like excitement can spread throughout a room.
It’s important to note that this doesn’t mean a gas can expand infinitely. If you try to squeeze too much gas into a container, like overinflating a tire, the pressure inside will increase. Eventually, the container might not be able to withstand the pressure and could burst. However, in most everyday scenarios, gases effectively take on the shape and volume of their container.
Understanding this property of gases is crucial in various fields. Designing a safe and efficient container for natural gas, for example, requires a deep understanding of how temperature and pressure affect the gas’s volume. Even comprehending weather patterns relies on knowledge of how these factors influence the volume of gases in our atmosphere.
Scientists are constantly making new discoveries about the behavior of matter. Still, next time you encounter a balloon, remember those energetic gas particles inside, bouncing around and claiming all the space they can get!
Does Water Have a Definite Volume?
Given that liquids tend to adopt the shape of their container, it’s natural to wonder: does water, the most ubiquitous liquid, truly have a set volume? It might seem counterintuitive, but the answer is yes!
Imagine water molecules as minuscule, constantly jiggling marbles, always bumping into each other. This “closeness,” maintained by intermolecular forces, is what gives liquids their definite volume. Even when we try to squeeze them into a smaller space, those molecules resist being pushed any closer together.
For instance, if you pour a cup of water (approximately 237 milliliters) into a tall, slender vase or a wide, shallow bowl, the water’s shape changes to match the container. However, the crucial point is that the total amount of water remains the same. This is because the volume, the actual space occupied by those tiny water molecules, stays constant.
Now, you might be thinking, “But ice is water, and it has a definite shape!” You’re right! Ice is unique because it’s the solid form of water. In solids like ice, the water molecules are locked into a fixed, organized structure, giving them both a defined shape and volume.
In contrast, gases like the air we breathe don’t have a fixed shape or volume. They expand to occupy whatever space is available to them.
The concept of liquid volume is surprisingly important. Consider cooking: many recipes call for precise measurements of liquid ingredients. In engineering, hydraulic systems, like those used in construction equipment, function based on the principle that liquids maintain a consistent volume.
Scientists continue to explore the properties of water and other liquids, making new discoveries all the time. While we have a good understanding of liquid volume today, further research might reveal even more about this essential property.
Do Liquids Have a Definite Density?
We’ve established that liquids, unlike gases, have a fixed volume. But what about their density? Is that also a constant property?
Imagine filling a glass with water. The water takes up a specific amount of space because its molecules are closely packed together, similar to a solid, albeit with more freedom of movement. This close arrangement is what gives liquids their definite volume.
Now, let’s heat that glass of water. You might observe the water level rising slightly because heat increases the kinetic energy of the molecules, making them move faster and spread out a little. However, even with this slight expansion, the overall volume change is relatively small. The same principle applies to pressure: applying pressure to a liquid might compress it slightly, but it won’t simply disappear.
Density is a measure of how much mass is contained within a given volume. Since liquids tend to maintain a consistent volume, they generally also have a relatively constant density. In fact, the densities of liquids are often quite close to those of solids!
This consistent volume and density make liquids incredibly useful. We rely on these properties in numerous applications, from the hydraulic systems in vehicles to the liquid medications we take. These substances are carefully formulated to behave in predictable ways, ensuring their effectiveness and reliability.
Of course, our understanding of liquids is always evolving. Scientists are constantly investigating the properties of liquids under more extreme conditions, such as exceptionally high temperatures and pressures. However, for most everyday situations, it’s safe to say that liquids do have a definite volume and density, making them essential components of our world.
Is Liquid Definite or Indefinite?
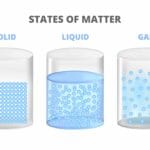
We’ve explored how solids prefer to maintain their shape, while gases spread out to fill any space they occupy. Now, let’s delve into the intriguing nature of liquids, which fall somewhere in between these two states of matter.
Liquids possess a definite volume, meaning they take up a specific amount of space. Pour yourself a glass of juice, and you know it will fill the glass to a certain level. This occurs because the liquid, in this case, juice, has a predetermined volume.
However, liquids are intriguing because they lack a fixed shape. They behave like chameleons of the container world! Pour that same juice into a tall glass, a wide bowl, or even an oddly shaped vase–the juice will effortlessly conform to the shape of its container. This adaptability stems from the nature of the tiny particles within liquids. While they are closely spaced, they still have the freedom to move around and slide past one another. This mobility allows liquids to flow and take on the form of whatever holds them.
The concept of a “definite volume” is surprisingly significant. Imagine trying to measure ingredients for baking without knowing how much space your liquids occupy–chaos in the kitchen! Engineers rely on the principle of fixed liquid volume when designing things like car engines, ensuring that fluids like oil operate within specific parameters.
However, it’s important to note that the “definite volume” of a liquid is not completely absolute. Temperature and pressure can influence a liquid’s volume to a small degree. For instance, heating a liquid generally causes it to expand slightly, resulting in a minor increase in volume. On the other hand, under extreme pressure, liquids can be compressed, meaning their volume can actually be reduced slightly.
Scientists are continuously investigating these nuances, delving deeper into how liquids behave under different conditions. So, while we have a good understanding of the fundamental principles, there’s always more to learn about these fascinating substances that flow and fill our world!
Is Volume Matter Yes or No?
Throughout this exploration of matter, we’ve discussed how substances can exist as solids, liquids, or gases. Now, let’s address a fundamental question: does volume really matter when it comes to liquids? The answer is a resounding YES!
Think about your favorite beverage, whether it’s juice, soda, or even water. When you pour it into a glass, does the amount of liquid magically change depending on the glass’s shape? Nope! The amount stays the same. This consistency is because liquids have a fixed volume.
It’s like a superpower: liquids can morph their shape to fit any container, but their total volume remains constant. They are the chameleons of the matter world!
This “fixed volume” phenomenon occurs because the particles within a liquid, while mobile, are still packed quite close together. They are free to move around and slide past each other, enabling liquids to flow and pour. However, they are close enough that squeezing them together to occupy less space is no easy feat.
Imagine trying to squeeze a water balloon. You can easily change its shape, but good luck trying to make the amount of water inside vanish! Liquids behave similarly.
This property of fixed volume might seem simple, but it has profound implications. Knowing that liquids maintain a consistent volume is crucial for a vast array of applications. Consider measuring ingredients in cooking, filling your car with gas, or even the operation of hydraulic systems–all rely on the predictable volume of liquids.
Scientists and engineers constantly uncover new ways in which the fixed volume of liquids impacts the world around us. There’s still much to explore about how this seemingly straightforward property influences complex systems and reactions.
So, to answer the question definitively, when it comes to liquids, volume definitely matters. It’s one of the defining characteristics that make liquids so fascinating and indispensable!
Can Liquid Be Compressed, Yes or No?
We’ve discussed the nature of liquids and how their particles behave differently than those in solids or gases. This difference in behavior raises an intriguing question: can you actually compress a liquid, squeezing it into a smaller space?
Think of a liquid like a bag already packed full of items. You might be able to rearrange things a bit, but fitting anything else in seems impossible. Similarly, the molecules in a liquid are already huddled closely together. There’s a little wiggle room, but not much. This close proximity explains why liquids take the shape of their container–the molecules simply flow into whatever space they’re given.
Now, imagine trying to cram even more items into that already overflowing bag. It’s going to be a challenge! The same applies to liquids. Those molecules are held together by intermolecular forces. They can nudge a bit closer, but try to squeeze them too tightly, and they will push right back. This resistance to compression is why we generally consider liquids to be incompressible. They really don’t appreciate being squished!
However, like most things in science, it’s not always so black and white. While liquids are almost entirely incompressible under normal circumstances, some scientists believe that under extreme pressure, even liquids might yield a tiny bit. We’re talking about pressures far beyond what we experience daily–think deep within the ocean or at the Earth’s core!
The concept of liquid compression is an ongoing topic of research, with scientists constantly developing new theories and conducting experiments. It’s possible that our understanding of liquids could evolve as we delve deeper into this area.
So, for most practical purposes, it’s safe to consider liquids as incompressible. However, it’s essential to remember that the universe is full of surprises, and there’s always more to discover when it comes to the wonders of science.
Does Air Have a Definite Volume: True or False?
We’ve been discussing matter and its various states: solids, with their fixed shape and volume; liquids, with their definite volume but changeable shape; and gases, which seem to behave quite differently. But does air, the most common gas we encounter, have a definite volume? The answer is false.
Imagine blowing up a balloon. As you exhale, you fill the balloon with air, causing it to expand. The air inside clearly occupies more space. Now, let the air out, and the balloon shrinks back down. This simple demonstration reveals that air’s volume is not fixed; it changes depending on the space it occupies.
Let’s break down why:
- Solids: Picture a tightly packed box of crayons. They have a specific shape and occupy a set amount of space. They have a definite volume.
- Liquids: Think of juice in a pitcher. It takes the shape of its container but retains the same volume. Pour it into a tall glass, and the shape changes, but the amount of juice remains constant.
- Gases: Envision those captivating glitter explosions. The glitter particles spread out to fill the entire space, regardless of its size. Air behaves similarly.
This difference in behavior boils down to the interactions between the particles in each state of matter. In solids, particles are tightly bound and have minimal freedom of movement. Liquids grant their particles a bit more freedom, allowing them to move around but remain relatively close together.
In contrast, gas particles are like free spirits, zipping around at high speeds with weak attractions to each other. They will naturally expand to fill any container they’re in, constantly bouncing off the walls.
This property of gases is why a balloon inflates when you blow into it and why a punctured tire deflates. Gases have neither a fixed shape nor a fixed volume. This characteristic makes them fascinating and somewhat unpredictable!
Understanding this fundamental property of gases is crucial in numerous applications, from designing gas storage containers to understanding weather patterns. Gases are an integral part of our world, and their ability to expand and contract plays a vital role in countless natural phenomena and technological advancements.
Important Points
- Liquids possess a definite volume, unlike gases, which expand to fill their container. This means that a specific amount of a liquid will always occupy roughly the same amount of space.
- The close packing of molecules in liquids contributes to their definite volume. These molecules are in constant motion but are close enough together that they resist being compressed into a smaller space.
- It’s important to remember that a liquid’s volume is not perfectly fixed. Heat can cause liquids to expand slightly, and extreme pressure can cause them to contract. However, for most everyday purposes, these changes are negligible.
- The consistent volume of liquids is essential for many applications. It allows us to make accurate measurements and is crucial for technologies like hydraulic systems.
- Scientists are still studying the intricacies of liquid behavior, especially under extreme conditions. As our understanding grows, we may discover even more about these fascinating substances.
Do you have any idea Is the volume of plasma definite or indefinite?
The volume of plasma is indefinite because it is not a fixed amount and can vary depending on factors such as hydration status, blood loss, and fluid intake.
- Red Cloud, NE: Discover Willa Cather’s Legacy - April 11, 2025
- Remember Old Social Media Sites? Their Rise and Fall - April 11, 2025
- How many days till Feb 3?Accurate Countdowns & Tools - April 11, 2025