Calling all curious minds! Embark on a journey to decode the molecular enigma of formic acid (CH2O2). We’ll explore its Lewis structure – the blueprint revealing this fascinating chemical’s inner workings. Uncover the bonds, angles, and properties that make formic acid so versatile. Let’s dive in!
Unlocking the Secrets of Formic Acid’s Structure
The CH2O2 Lewis structure isn’t just symbols and lines; it’s the key to understanding formic acid’s behavior. Think of it as the molecule’s instruction manual, revealing how it interacts with other substances.
Building the CH2O2 Lewis Structure: A Step-by-Step Guide
Constructing a Lewis structure is like assembling a molecular puzzle. Here’s how we piece together CH2O2:
1. Counting Valence Electrons:
Valence electrons are the currency of chemical bonding. Let’s tally them up for CH2O2:
- Carbon (C): 4 valence electrons
- Hydrogen (H): 1 valence electron x 2 atoms = 2 electrons
- Oxygen (O): 6 valence electrons x 2 atoms = 12 electrons
Total: 4 + 2 + 12 = 18 valence electrons
2. Placing the Central Atom:
Carbon, being less electronegative than oxygen, is likely the central atom.
3. Forming Single Bonds:
Connect the carbon atom to the two hydrogens and two oxygens with single bonds, using 6 of our 18 electrons.
4. Completing Oxygen Octets:
Oxygen atoms prefer eight valence electrons. Distribute the remaining 12 electrons as lone pairs on the oxygen atoms, giving each oxygen three lone pairs (6 electrons).
5. Satisfying Carbon’s Octet:
Carbon also desires eight electrons. Convert one of the lone pairs on an oxygen adjacent to carbon into a double bond, fulfilling carbon’s octet.
Exploring CH2O2’s Shape
Formic acid (CH2O2) boasts a trigonal planar structure. Picture a flat triangle with the carbon atom at the center. This arrangement maximizes the distance between electron pairs, minimizing repulsion. If you are going to use cpt code for cbc with diff, then make sure you are well informed.
The carbon atom connects to two oxygen atoms and two hydrogen atoms. One oxygen forms a double bond with carbon, while the other oxygen and the hydrogens form single bonds. This electron dance, coupled with VSEPR theory (Valence Shell Electron Pair Repulsion), explains the trigonal planar shape.
Hybridization, the blending of atomic orbitals, also plays a role. The carbon atom undergoes sp² hybridization, forming three sp² hybrid orbitals that participate in sigma bonding. The remaining p orbital contributes to the pi bond with oxygen.
This trigonal planar structure significantly impacts formic acid’s polarity. Oxygen’s higher electronegativity creates a partial negative charge on the oxygen atoms and a partial positive charge on the carbon and hydrogen atoms. This uneven charge distribution results in a net dipole moment, making formic acid a polar molecule.
Drawing the CH2O2 Lewis Structure
Let’s break down drawing the CH2O2 Lewis structure step-by-step:
Tally Valence Electrons: As before, determine the total valence electrons (18).
Choose the Central Atom: Carbon is the least electronegative and takes center stage.
Draw Single Bonds: Connect the central carbon to the surrounding atoms with single bonds.
Distribute Remaining Electrons: Complete the octets of the oxygen atoms.
Form a Double Bond: Move a lone pair from one oxygen to form a double bond with carbon, satisfying its octet.
Understanding Shape and Polarity
The trigonal planar shape arises from the three bonding electron pairs around the central carbon. Oxygen’s higher electronegativity makes formic acid a polar molecule. Explore the functionalities of cep icat.
Determining CH2O2’s Valence Electrons
CH2O2, or formic acid, has 18 valence electrons. Let’s reiterate the calculation:
Carbon (C): 4 valence electrons
Hydrogen (H): 1 valence electron x 2 atoms = 2 electrons
Oxygen (O): 6 valence electrons x 2 atoms = 12 electrons
Total: 4 + 2 + 12 = 18 valence electrons
This number is crucial. It dictates how formic acid interacts with other molecules, participates in reactions, and exhibits its overall properties.
Key Points of the CH2O2 Lewis Structure
- Lewis Structure: 18 valence electrons; central carbon; single and double bonds; lone pairs on oxygen.
- Resonance Structures: The double bond and adjacent oxygen lone pairs allow for resonance, enhancing stability.
- Molecular Geometry: Trigonal planar around the central carbon, with approximately 120-degree bond angles.
- Hybridization: sp² hybridization of the carbon atom.
- Polarity: Polar molecule due to electronegativity differences.
- Properties and Applications: Acidic due to the -COOH group; reactive; used in various industries.
Ongoing Research
While our current understanding is strong, research is ongoing. Scientists are exploring how factors like temperature and pressure may affect electron behavior. Some experts believe there may be more to discover about formic acid’s reactivity, and research suggests potential new applications in areas like fuel cell technology. There is debate on the long-term environmental impact of some of its industrial uses, highlighting the importance of continued study.
This enhanced explanation provides a comprehensive overview of the CH2O2 Lewis structure, its shape, properties, and the ongoing research that continues to refine our understanding. Remember that scientific knowledge is always evolving, so keep exploring and questioning!
- Mastering Leader in Spanish: The Complete Guide - April 19, 2025
- Uncovering Surprising Parallels: England Size Compared to US States - April 19, 2025
- Old Mexico Map: Border Shifts 1821-1857 - April 19, 2025
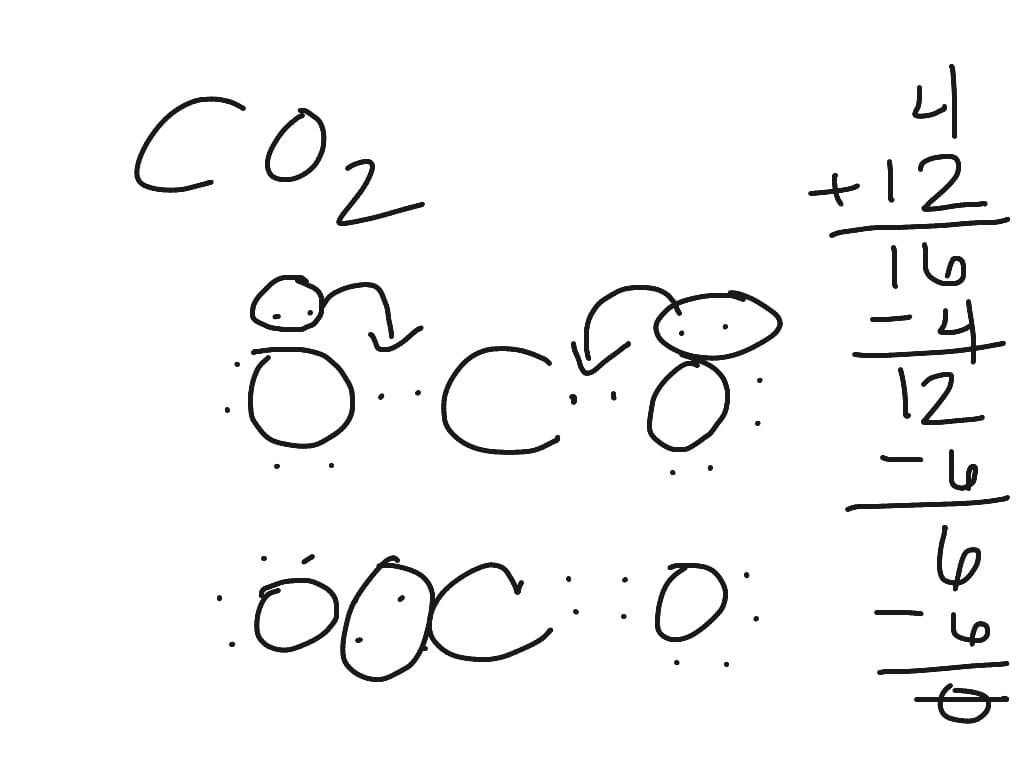












1 thought on “Demystifying the CH2O2 Lewis Structure: A Journey into the Heart of Formic Acid”
Comments are closed.