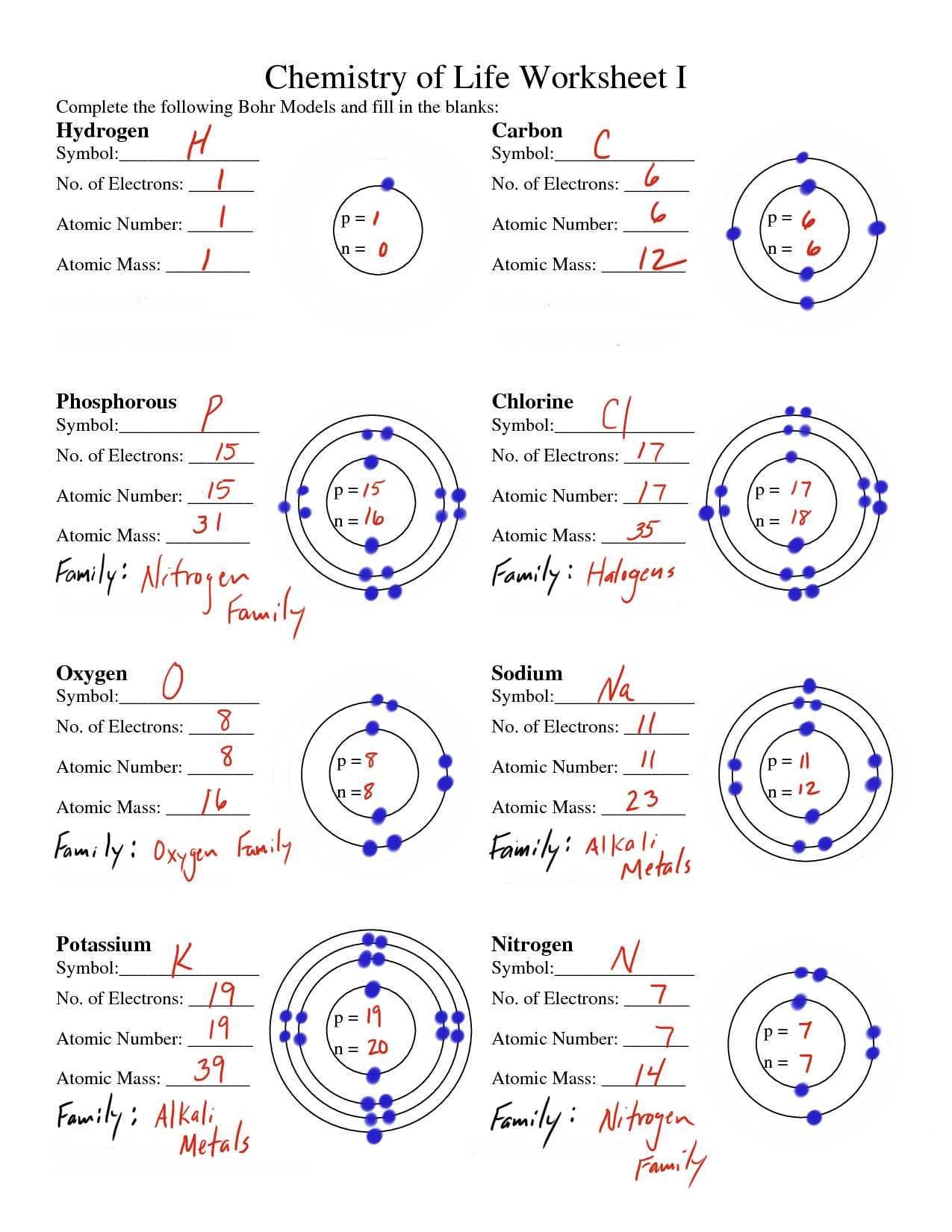
Get ready to explore the fascinating world of atoms! These tiny structures, composed of protons, neutrons, and electrons, are the fundamental building blocks of everything around us. Think of an atom like a microscopic solar system, with the nucleus at the center (like the sun) and electrons orbiting around it (like planets). This guide will break down atomic structure, from its basic components to its intricate organization, making you an atom expert in no time!
Deciphering the Atom: Basic Structure and Key Terms
Imagine an atom as a minuscule solar system. At its core lies the nucleus, home to protons (positively charged particles) and neutrons (neutral particles). Zipping around the nucleus are electrons, incredibly small particles carrying a negative charge.
Important Concepts:
- Atomic Number: An atom’s ID number! It tells you how many protons an atom has.
- Mass Number: Add up the protons and neutrons to get the mass number. It’s like weighing the atom’s nucleus.
- Isotopes: Atoms of the same element can have different numbers of neutrons, these are called isotopes. Think of them as atomic siblings.
The Electron Dance: Unraveling Electron Configuration
Electrons don’t just randomly buzz around the nucleus; they inhabit specific energy levels, similar to floors in a building. These levels are called shells and subshells. The way electrons are arranged in these levels is known as their electron configuration.
Here’s a breakdown:
- Shells: The main energy levels of an atom.
- Subshells: Within each shell, there are subshells (orbitals) with slightly different energy levels. Each subshell has a unique shape.
- Quantum Numbers: Think of these as the electron’s address within the atom. Quantum numbers specify the shell, subshell, orientation, and spin of an electron.
The Periodic Table Connection:
The arrangement of elements in the periodic table isn’t random! It’s based on electron configuration patterns. Elements in the same vertical column (group) often share similar chemical properties due to similarities in their electron arrangements.
Test Your Knowledge: Worksheets and Answer Keys
Ready to put your newfound atomic knowledge to the test? Our worksheets [link to downloadable worksheets] are designed to solidify your understanding:
- Diagram Labeling: Identify and label the protons, neutrons, and electrons in atomic diagrams.
- Fill-in-the-Blanks: Test your recall of key terms and concepts.
- And More! Explore a variety of exercises to reinforce your grasp of atomic structure.
Don’t worry, you’re not alone! Detailed answer keys [link to answer keys] are provided for self-assessment and to help you learn from any mistakes.
Delve Deeper: Additional Resources for Atomic Exploration
Eager to journey further into the captivating world of atoms? Check out these resources:
- Interactive Simulations: Witness atomic structure in action through dynamic simulations [link to recommended simulations].
- Engaging Videos: Learn from experts in the field through captivating video explanations [link to recommended videos].
- Interactive Periodic Tables: Explore the elements and their properties in an immersive way [link to recommended interactive periodic tables].
Unlocking Atomic Structure: A Step-by-Step Guide
Let’s break down how to determine the structure of an atom, using what we’ve learned:
1. Identify the Element:
Start with the element’s name or symbol (e.g., Oxygen, O).
2. The Atomic Number is Key:
The atomic number (Z), found on the periodic table, reveals the number of protons in the atom’s nucleus. Each element has a unique atomic number.
3. Neutron Sleuthing:
To find the number of neutrons, you’ll need the element’s mass number (A), usually a decimal number on the periodic table. Round the mass number to the nearest whole number and then subtract the atomic number (number of protons):
Neutrons = Mass Number – Atomic Number
4. Electrons in Orbit (Sort Of):
In a neutral atom, the number of electrons is equal to the number of protons. These electrons occupy specific energy levels (shells) around the nucleus.
Example: Deciphering Carbon
Let’s unravel the atomic structure of Carbon (C):
- Element: Carbon (C)
- Atomic Number (Z): 6 (from the periodic table), meaning Carbon has 6 protons.
- Mass Number (A): 12.011 (from the periodic table). Round to 12.
- Neutrons = 12 – 6 = 6 neutrons
- Electrons: Carbon has 6 electrons (same as the number of protons).
Therefore, a carbon atom has 6 protons, 6 neutrons, and 6 electrons.
Beyond the Textbook: The 4 Models That Shaped Our Understanding of Atomic Structure
Our understanding of the atom, once considered an indivisible sphere, has evolved dramatically over centuries of scientific exploration. Four major models mark this fascinating journey:
1. The Indivisible Atom (Ancient Greece – 19th Century):
* Key Figures: Democritus, John Dalton
* Core Idea: Matter comprises tiny, indivisible particles called atoms, considered fundamental and unbreakable.
* Limitations: Failed to explain observations like static electricity or chemical reactions, which hinted at an internal structure within atoms.
2. The Plum Pudding Model (1904):
* Key Figure: J.J. Thomson
* Core Idea: The atom is envisioned as a positively charged “pudding” with negatively charged electrons embedded throughout, like plums in a pudding.
* Experimental Basis: The discovery of electrons, through experiments with cathode rays, challenged the idea of an indivisible atom.
* Limitations: Couldn’t explain the arrangement of charges within the atom or the results of later scattering experiments.
3. The Nuclear Model (1911):
* Key Figure: Ernest Rutherford
* Core Idea: The atom has a tiny, dense, positively charged nucleus at its center, with electrons orbiting around it like planets around the sun.
* Experimental Basis: Rutherford’s gold foil experiment, where alpha particles were deflected at various angles when fired at a thin gold sheet, provided evidence for a small, dense nucleus.
* Limitations: This model couldn’t explain why electrons didn’t spiral into the nucleus or the specific wavelengths of light emitted by excited atoms (atomic spectra).
4. The Quantum Mechanical Model (1920s – Present):
* Key Figures: Niels Bohr, Erwin Schrödinger, Werner Heisenberg
* Core Idea: Electrons don’t follow fixed orbits but exist in specific energy levels, or orbitals, around the nucleus. These orbitals are regions of probability, where an electron is likely to be found.
* Experimental Basis: Spectroscopy (the study of light emitted and absorbed by atoms), wave-particle duality (the concept that electrons can behave like both particles and waves)
* Significance: This is the most accurate and sophisticated atomic model, forming the foundation of modern chemistry and our understanding of the behavior of matter.
What are the 3 Rules of Atomic Structure? Unveiling the Atom’s Secrets
Three fundamental rules govern the arrangement of electrons within an atom, dictating its chemical properties and reactivity:
1. The Aufbau Principle:
* The Rule: Electrons fill atomic orbitals in order of increasing energy, starting with the lowest energy levels closest to the nucleus.
* Think of it like: Filling a building with people, starting from the ground floor and moving upward only when lower floors are full.
* Why it matters: Electrons are attracted to the positively charged nucleus. Orbitals closer to the nucleus have lower energy, making them more stable and preferable for electrons to occupy first.
2. Hund’s Rule:
* The Rule: When electrons occupy orbitals with the same energy (degenerate orbitals), they spread out across these orbitals with parallel spins (all spin-up or all spin-down) before pairing up in the same orbital.
* Think of it like: Students choosing desks in an empty classroom. They’ll likely spread out to maximize space before doubling up at the same desk.
* Why it matters: Electrons, being negatively charged, repel each other. Spreading out in separate orbitals with parallel spins minimizes electron-electron repulsion, leading to a more stable electronic configuration.
3. The Pauli Exclusion Principle:
* The Rule: No two electrons in an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (one spin-up, one spin-down).
* Think of it like: A parking space designed for only two cars. Each car has a specific spot and must face a designated direction (representing opposite spins).
* Why it matters: This principle ensures that each electron within an atom has a unique quantum state, characterized by its energy level, orbital shape, spatial orientation, and spin.
Conclusion:
You’ve now embarked on a journey into the heart of matter, exploring the fundamental building blocks of our universe! Understanding atomic structure and the rules governing electron behavior unlocks a deeper comprehension of chemistry, physics, and the natural world. As you continue your exploration, remember that scientific inquiry is an ongoing process, and new discoveries about the atom may yet await!
Internal Links:
- Uncover the astonishing grandeur of Colorado’s mountains at altitude Ouray, where breathtaking vistas await.
- Embark on a linguistic adventure with antitoxins crossword and unravel the mysteries hidden within the grid.