Symmetry isn’t just aesthetically pleasing; it’s a fundamental principle governing molecular properties and behavior. This guide delves into the C2v point group, a common type of molecular symmetry, and its associated character table—an essential tool for chemists and spectroscopists. We’ll explore the table’s structure, interpret its elements, and illustrate its practical applications with clear examples, empowering you to unlock valuable insights into the molecular world.
Identifying C2v Symmetry: Spotting the Clues
How can you tell if a molecule belongs to the exclusive C2v symmetry club? It’s like recognizing a familiar face in a crowd; you’re looking for specific features. Imagine a molecule floating in 3D space. Can you rotate it 180 degrees around an axis and have it look exactly the same? If so, you’ve likely found a C2 rotation axis. Now, picture two vertical mirror planes (σv) intersecting along this axis. Do reflections in these planes produce identical images of the original molecule? If the molecule passes both tests, it likely possesses C2v symmetry. Water (H2O) and formaldehyde (CH2O) are classic examples—visualize them and you’ll probably see the symmetry elements at play. Check our detailed USCS soil classification chart to see other instances of C2v symmetry examples.
Decoding the C2v Character Table: Rows, Columns, and Characters
The C2v character table might appear intimidating at first, but it’s essentially a well-organized summary of how a molecule’s symmetry influences its properties.
Rows (Irreducible Representations): Think of these rows, labeled A1, A2, B1, and B2, as distinct molecular personalities. These symbols, called irreducible representations, categorize the fundamental ways a molecule’s components can transform under symmetry operations.
Columns (Symmetry Operations): The columns represent the symmetry operations we can perform: E (doing nothing—the identity operation), C2 (180-degree rotation), σv(xz) (reflection across the xz plane), and σv(yz) (reflection across the yz plane).
Characters (The Numbers): At the intersection of each row and column, you’ll find a character (+1 or -1). These numbers indicate how each “personality” (irreducible representation) reacts to a particular symmetry operation. A +1 means the property remains unchanged, while a -1 signifies a sign flip.
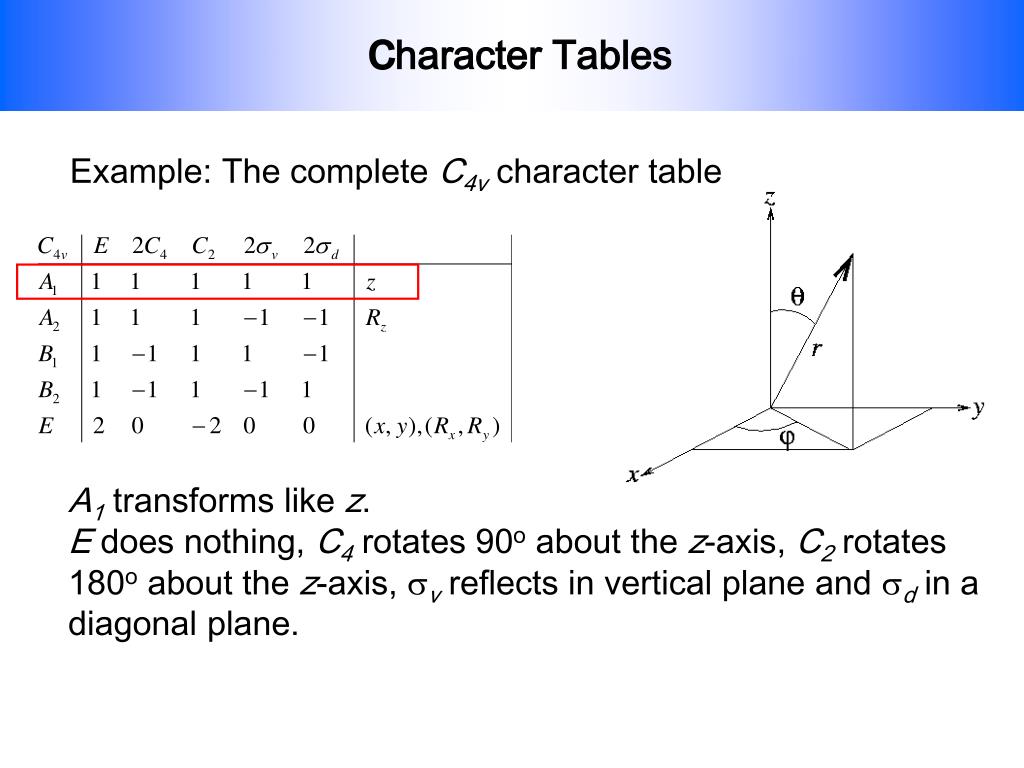
Here’s a typical C2v character table:
| C2v | E | C2 | σv(xz) | σv(yz) |
|---|---|---|---|---|
| A1 | +1 | +1 | +1 | +1 |
| A2 | +1 | +1 | -1 | -1 |
| B1 | +1 | -1 | +1 | -1 |
| B2 | +1 | -1 | -1 | +1 |
Applying the C2v Character Table: Unveiling Molecular Secrets
The power of the C2v character table lies in its predictive capabilities. Let’s explore some key applications:
1. Predicting Spectroscopic Activity: Illuminating Molecular Vibrations
How does the table help us understand a molecule’s interactions with light? Each vibrational mode of a molecule transforms according to a specific irreducible representation. The C2v character table reveals which of these vibrations are “IR active” or “Raman active”—meaning they absorb or scatter light at specific frequencies. For example, a vibration transforming like x, y, or z (as indicated alongside certain irreducible representations in some tables) is IR active. Similarly, Raman activity is often linked to transformations mirroring quadratic functions (x2, y2, z2, xy, xz, yz). This information allows us to predict and interpret the unique patterns observed in a molecule’s spectrum, much like decoding a fingerprint. Optimize your analysis further with effective SMLM tracking to visualize these molecular vibrations.
2. Constructing Molecular Orbitals: Building with Symmetry
Imagine atomic orbitals as building blocks for molecules. When atoms combine, their orbitals interact to create molecular orbitals. Symmetry acts as a strict architect, dictating which combinations are allowed. The C2v character table guides us in this molecular construction, revealing which atomic orbitals can combine to form specific molecular orbitals based on their symmetry properties. For example, in water, the hydrogen 1s orbitals can combine symmetrically (A1) or antisymmetrically (B2), each interacting differently with oxygen’s orbitals.
3. Exploring Further Applications: Beyond the Basics
The C2v character table’s applications extend beyond spectroscopy and molecular orbitals. It can provide insights into:
- Molecular Polarity: Symmetry influences whether a molecule has a positive and negative end (polarity), affecting its interactions with other molecules.
- Reactivity: The table can offer clues about how a molecule might react, as certain symmetry properties can facilitate or hinder chemical transformations.
- Quantum Chemistry Calculations: Advanced software tools use symmetry principles derived from character tables to simplify complex quantum chemical calculations, enabling researchers to study molecular behavior in greater detail.
Advanced Concepts and Ongoing Research: Expanding Our Understanding
While this guide provides a solid foundation, the field of molecular symmetry is constantly evolving. Ongoing research explores topics like:
- Symmetry Breaking: What happens when a molecule’s ideal symmetry is slightly distorted? How does this affect its properties?
- Complex Systems: How do symmetry principles apply to larger, more intricate molecules and molecular assemblies?
- Time-Dependent Symmetry: How do we describe the symmetry of dynamic processes, such as chemical reactions?
Current research suggests that even subtle deviations from perfect symmetry can significantly impact molecular properties and behavior. This highlights the importance of continued exploration and the potential for future discoveries.
Conclusion: Embracing the Power of Symmetry
The C2v character table, and the principles of symmetry it represents, are indispensable tools for chemists and spectroscopists. By understanding and applying these concepts, we gain powerful insights into the structure, properties, and reactivity of molecules. While our understanding is already extensive, there is still much to learn. Ongoing research continues to uncover the subtle yet profound influence of symmetry in the molecular world.
- China II Review: Delicious Food & Speedy Service - April 17, 2025
- Understand Virginia’s Flag: History & Debate - April 17, 2025
- Explore Long Island’s Map: Unique Regions & Insights - April 17, 2025