Did you know the sweltering stretch of summer we often experience is actually the hot summer period named for Sirius? And for those seeking eco-friendly cooling solutions, understanding the properties of isobutane R600a refrigerant is becoming increasingly important. Just as we can predict the hottest days of summer, we can also predict the genetic makeup of populations with the help of a fascinating tool from population genetics: the Hardy-Weinberg principle. This guide serves as your comprehensive introduction to this powerful tool and how to use a Hardy-Weinberg calculator.
Decoding the Hardy-Weinberg Principle
The Hardy-Weinberg principle provides a baseline for understanding how gene variants, called alleles, are distributed within a population. Imagine a vast pool of genes, much like a giant jar of red and blue marbles. The principle suggests that, under specific conditions, the proportions of these “marbles” (alleles) and the combinations they form (genotypes) will remain relatively stable across generations. It’s a snapshot of a population’s genetic makeup if no evolutionary forces were acting upon it.
This stability, however, hinges on five key assumptions, often referred to as the “five pillars” of Hardy-Weinberg equilibrium:
- Absence of Mutation: No new alleles are arising.
- Random Mating: Mating choices are not influenced by genetic traits.
- No Gene Flow: There’s no migration of individuals into or out of the population.
- No Genetic Drift: The population is large enough to avoid random fluctuations in allele frequencies.
- No Natural Selection: No allele confers a survival or reproductive advantage.
In real-world populations, these conditions are rarely fully met. However, this theoretical ideal is invaluable because deviations from Hardy-Weinberg equilibrium provide strong evidence that evolutionary forces are influencing the population.
Unraveling the Hardy-Weinberg Equation
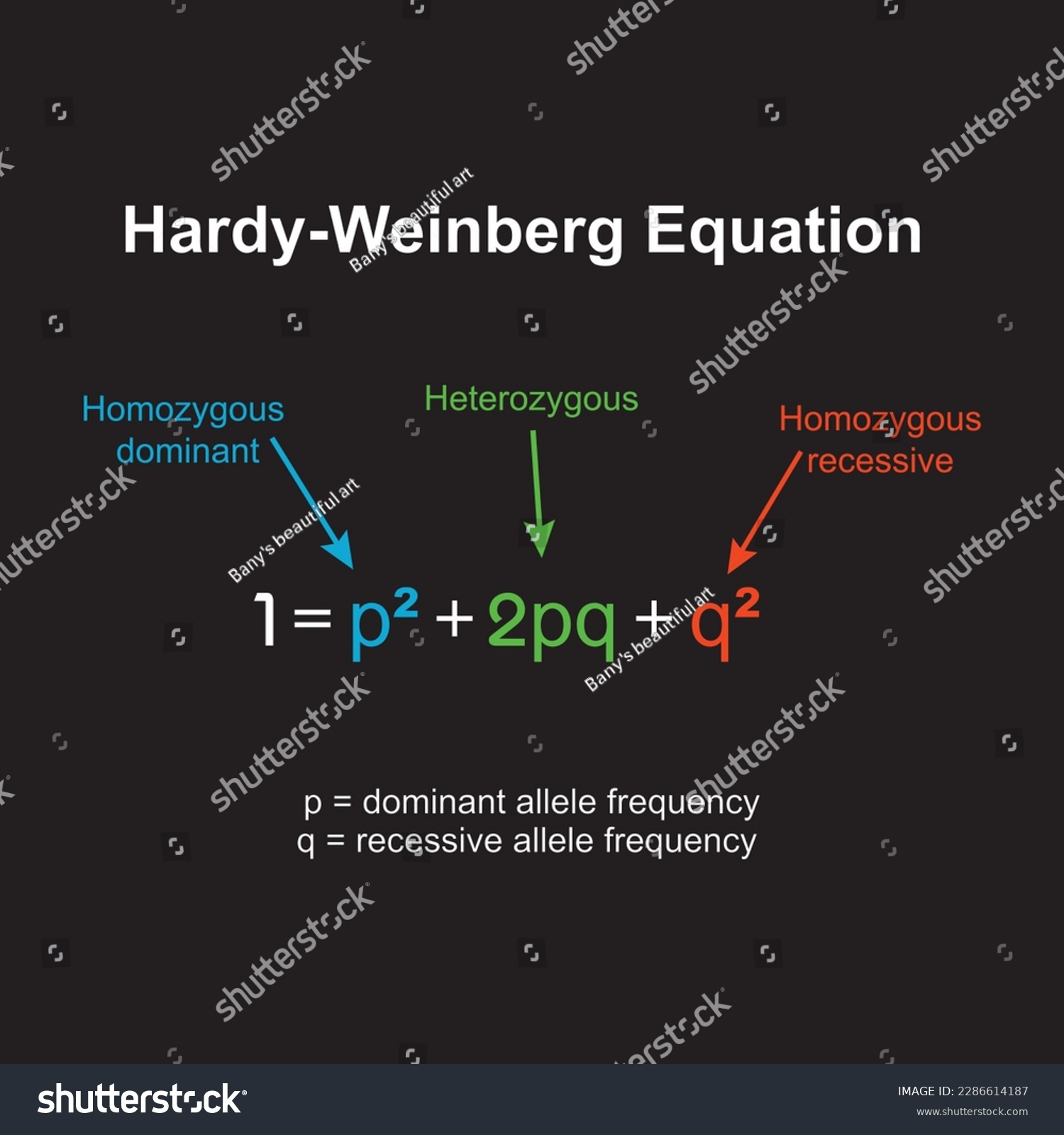
At the heart of the Hardy-Weinberg principle lies a fundamental equation: p² + 2pq + q² = 1. This equation links the frequency of alleles to the frequency of genotypes within a population. Let’s break it down:
- p: Frequency of the dominant allele (often denoted as ‘A’). This allele’s trait is typically expressed even if only one copy is present.
- q: Frequency of the recessive allele (often denoted as ‘a’). This allele’s trait is expressed only when two copies are present.
- _p_²: Frequency of individuals with two dominant alleles (homozygous dominant, AA).
- 2_pq_: Frequency of individuals with one dominant and one recessive allele (heterozygous, Aa). These individuals are often called “carriers” of the recessive allele.
- _q_²: Frequency of individuals with two recessive alleles (homozygous recessive, aa).
Because there are only two alleles in our simplified model, their frequencies must add up to 1 (or 100%): p + q = 1.
Navigating Hardy-Weinberg Calculators
Hardy-Weinberg calculators simplify the application of the principle. Several types of calculators cater to different needs:
- Basic Calculators: Ideal for scenarios with two alleles. These calculators can determine all genotype frequencies if you know either p or q. Examples include calculators found on scienceprimer.com, wpcalc.com, and geneswellness.com.
- Advanced Calculators: Designed for more complex scenarios, like when a gene has multiple alleles. Some advanced calculators, such as the one on biocompute.org.uk, also correct for potential biases in data collection.
- Specialized Calculators: Tailored for specific applications. For example, perinatology.com and omnicalculator.com offer calculators that estimate carrier frequencies for recessive genetic disorders.
Applying the Hardy-Weinberg Principle: Real-World Examples
The Hardy-Weinberg principle has far-reaching applications:
- Predicting Disease Risk: By knowing the frequency of a disease-causing recessive allele (q), we can estimate the carrier frequency (2_pq_), which is crucial for genetic counseling and public health initiatives.
- Unraveling Evolutionary Forces: Deviations from Hardy-Weinberg equilibrium suggest that evolutionary pressures like natural selection, genetic drift, or gene flow are at play.
- Aiding Conservation Efforts: By analyzing allele frequencies, conservationists can assess the genetic diversity of endangered populations and develop strategies to protect them.
Example: Cystic Fibrosis Carrier Frequency
Cystic fibrosis is a recessive genetic disorder. Let’s assume the frequency of the cystic fibrosis allele (q) is 0.02 in a particular population.
- Calculate p: Since p + q = 1, p = 1 – 0.02 = 0.98.
- Calculate carrier frequency (2_pq_): 2 * 0.98 * 0.02 = 0.0392. This suggests that approximately 3.92% of the population are carriers for cystic fibrosis.
The Limits of the Model
While powerful, the Hardy-Weinberg principle represents an idealized scenario. Real populations rarely adhere strictly to its assumptions. Factors like mutations, migration, non-random mating, and natural selection introduce deviations from the expected equilibrium. Ongoing research continues to refine our understanding of how these factors interact and influence the genetic diversity of populations.
Some scientists debate the relative contributions of different evolutionary forces to observed deviations from the Hardy-Weinberg equilibrium, highlighting the dynamic nature of this field of study. While the principle offers a valuable framework, it’s crucial to interpret its results with an awareness of its limitations. Using cautious language, acknowledging uncertainties, and presenting different perspectives when discussing the nuances of Hardy-Weinberg can significantly improve the accuracy and credibility of scientific communication.