Understanding the helium Lewis dot structure unlocks the secrets of this unique element’s stability and inertness. This guide provides a step-by-step explanation, complete with diagrams and FAQs, to help you master this fundamental concept in chemistry. Whether you’re a student, a science enthusiast, or just curious about the world around you, this guide is your key to understanding why helium behaves the way it does. For those who appreciate artistry and cultural expression, explore our collection of kufiyah.
Deciphering Helium’s Atomic Structure
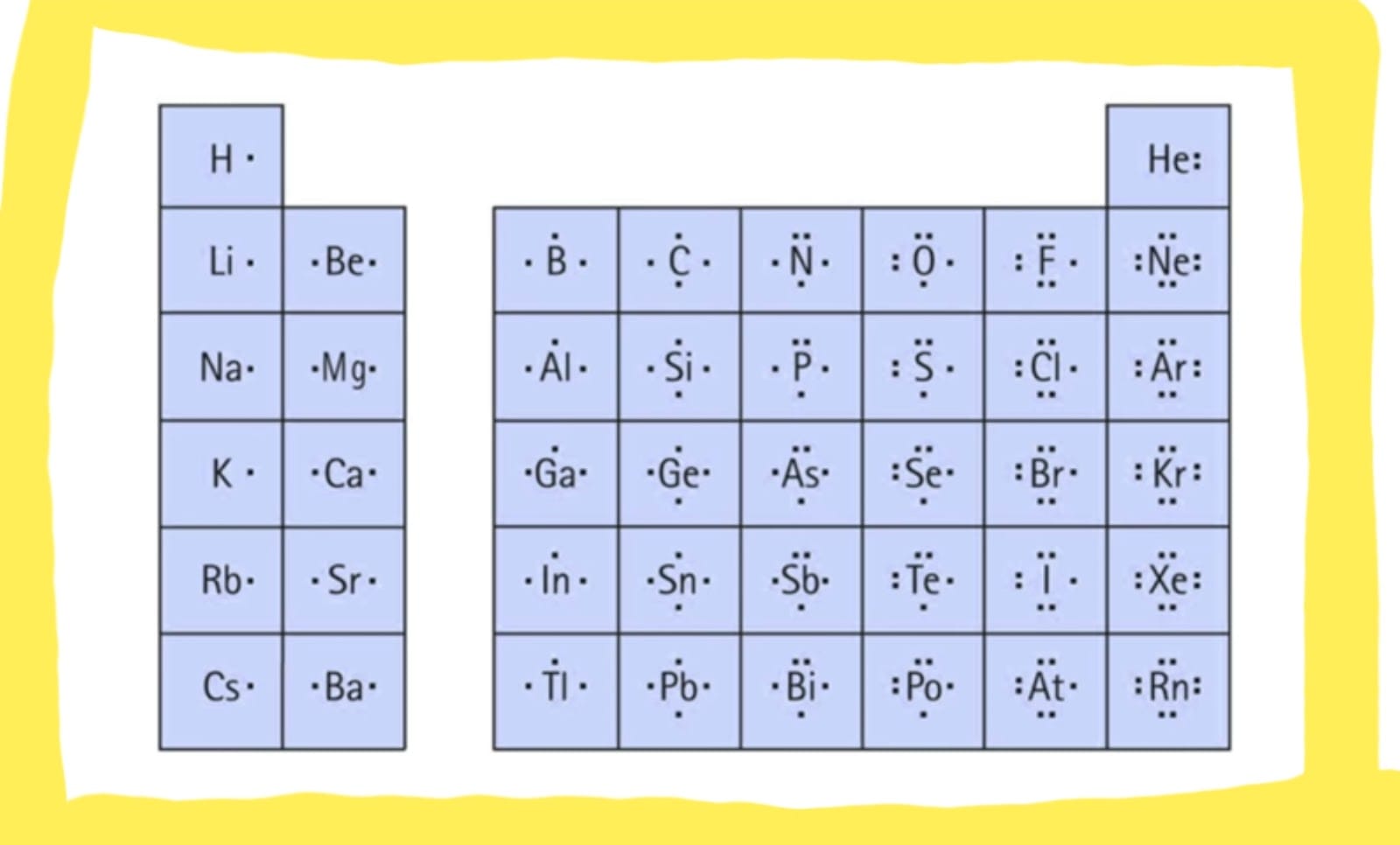
Before diving into Lewis dot structures, let’s quickly recap what makes helium so special. With an atomic number of 2, helium has two protons and two electrons. These two electrons are helium’s valence electrons – the outermost electrons responsible for its chemical behavior. Unlike most atoms that strive for eight valence electrons (the octet rule), helium is content with its two. This seemingly small detail has profound implications for its properties. Helium’s electron configuration, 1s², tells us that both electrons comfortably reside in the first and only electron shell, making it exceptionally stable. If you’re interested in historical properties, the litchfield villa brooklyn department of parks & recreation is a great resource.
Drawing the Helium Lewis Dot Structure: A Visual Guide
Representing helium’s electron arrangement visually is surprisingly straightforward. Lewis dot structures provide a simple yet powerful way to depict valence electrons. Here’s how to draw helium’s:
The Symbol: Start by writing “He,” the element symbol for helium. This represents the nucleus and any inner electrons (which helium doesn’t have).
The Dots: Helium has two valence electrons. Represent each one with a dot next to the symbol. While you could place them on opposite sides, convention typically places them together, like this: He:. This pairing visually reinforces the concept of the electrons residing in the same orbital.
That’s it! He: is the complete Lewis dot structure for helium. This simple diagram speaks volumes about helium’s chemical behavior.
Why Helium is a “Lone Wolf”: Understanding its Inertness
Helium’s two valence electrons form a lone pair, filling its outermost shell. This full shell is the key to helium’s inertness, its reluctance to participate in chemical reactions. It doesn’t need to gain, lose, or share electrons with other atoms, making it incredibly stable.
Helium’s Superpowers: Applications of Inertness
Helium’s inertness isn’t just a theoretical curiosity; it translates into a range of real-world applications:
- Balloons: Helium’s low density and non-reactivity make it a safer alternative to hydrogen for lifting balloons.
- Cryogenics: Its extremely low boiling point makes it essential for supercooling materials in various scientific and industrial processes.
- Leak Detection: Helium’s small atomic size allows it to escape through tiny cracks, making it ideal for detecting leaks in sealed systems.
- Welding: It provides an inert atmosphere, protecting welds from reactive gases in the air.
- MRI Machines: Helium cools the superconducting magnets crucial for MRI technology.
FAQs: Delving Deeper into Helium’s Lewis Dot Structure
Why is Helium’s Lewis Dot Structure Different from Hydrogen’s?
Hydrogen (H) has only one valence electron, represented by a single dot (H•) in its Lewis dot structure. This single electron makes hydrogen highly reactive, eager to pair up with another electron from a different atom. Helium, with its complete valence shell (He:), is content and unreactive.
Does Helium Have a Lone Pair?
Yes, helium’s two valence electrons form a lone pair. This lone pair resides in the 1s orbital, filling helium’s outermost shell and contributing to its stability and inertness.
What Does 1s² Mean?
1s² is helium’s electron configuration. It signifies that both of helium’s electrons occupy the 1s orbital, the lowest energy level in an atom. The superscript “2” indicates that two electrons reside in this orbital.
The Future of Helium Research
While our understanding of helium’s behavior is well-established, research continues. Scientists explore its properties under extreme conditions, pushing the boundaries of our knowledge. While helium’s Lewis dot structure and inherent stability are unlikely to change, ongoing research may reveal subtle nuances in its behavior, further enriching our understanding of this remarkable element.
- Unlock Elemental 2 Secrets: Actionable Insights Now - April 2, 2025
- Lot’s Wife’s Name: Unveiling the Mystery of Sodom’s Fall - April 2, 2025
- Photocell Sensors: A Complete Guide for Selection and Implementation - April 2, 2025