Hey there, science explorers! Ever wondered about the secrets hidden within a tiny molecule like thiocyanate (SCN-)? It’s like a miniature chemical orchestra, with electrons constantly moving and interacting. Join me as we unravel the mysteries of the SCN- Lewis structure, its shape, and how it all connects to its chemical behavior. It’s more exciting than you might think!
Decoding the SCN- Lewis Structure
Let’s start by building the Lewis structure of SCN-, a sort of molecular blueprint that shows us how the atoms are connected and where the electrons hang out. Think of sulfur (S), carbon (C), and nitrogen (N) as our main players, with an extra electron giving the whole group a negative charge.
Building the Blueprint: A Step-by-Step Guide
Constructing a Lewis structure is like assembling a puzzle. Here’s how we put together the SCN- puzzle:
Counting Valence Electrons: Each atom brings a certain number of valence electrons (the outermost electrons involved in bonding). Sulfur has 6, carbon has 4, and nitrogen has 5. Plus, we have that extra electron (for the negative charge), giving us a total of 16 valence electrons.
Connecting the Atoms: Carbon usually takes center stage in these molecules, so we’ll put it in the middle, bonded to sulfur and nitrogen with single bonds. Each single bond represents two shared electrons.
Completing Octets: Atoms generally prefer a full outer electron shell (an octet, or 8 electrons, except for hydrogen). We’ll add lone pairs (electrons not involved in bonding) to sulfur and nitrogen until they each have eight electrons.
Double Bonds to the Rescue: Carbon only has four electrons at this point. To give it a full octet, we’ll upgrade the single bonds to double bonds. This means sulfur and nitrogen each share two pairs of electrons with carbon, often leading to a more stable structure.
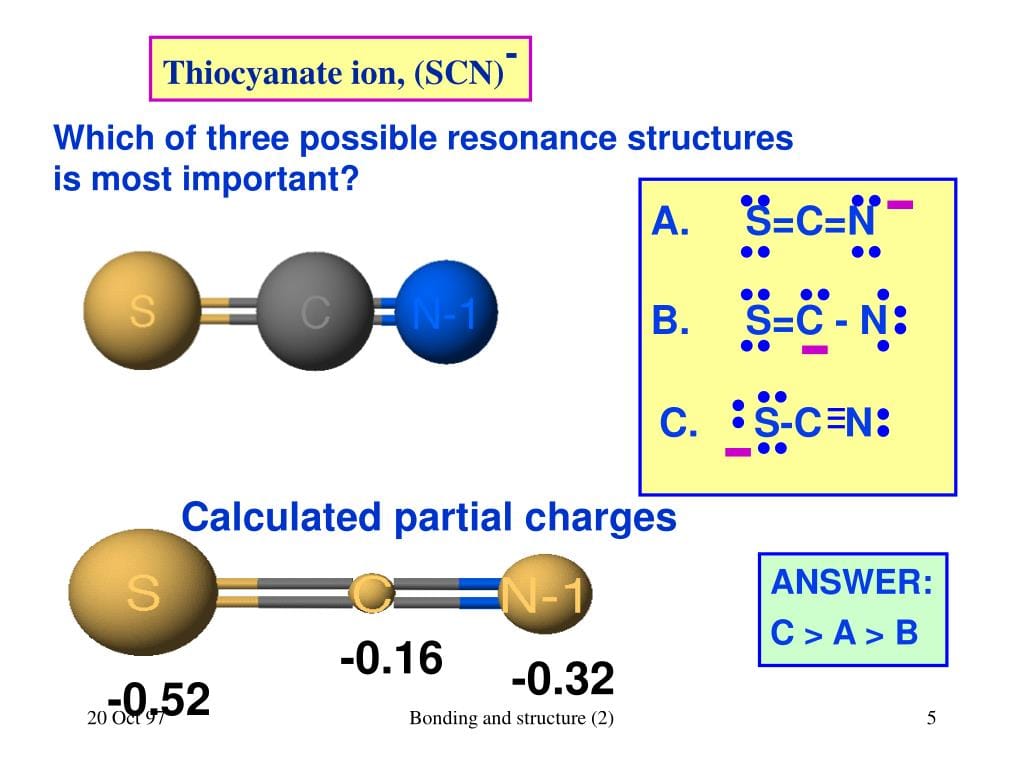
Resonance: The SCN- Shape-Shifter
SCN- is a bit of a shape-shifter, exhibiting resonance. This means there are multiple valid Lewis structures we can draw. The double bonds can shift around, and the negative charge can reside on either the sulfur or the nitrogen. Think of it as electrons playing musical chairs! While several structures are possible, the one with the negative charge on nitrogen is generally the most stable, likely due to nitrogen’s higher electronegativity (its stronger pull on electrons).
SCN- in 3D: Geometry and Hybridization
The Lewis structure gives us a 2D view, but molecules exist in 3D! The SCN- ion has a linear molecular geometry—a straight line. The central carbon atom is sp hybridized, meaning its electron orbitals have rearranged to form two hybrid orbitals participating in the double bonds.
Why the SCN- Lewis Structure Matters
Understanding the SCN- Lewis structure helps us predict how SCN- might react with other molecules. It’s also essential in coordination chemistry, where SCN- acts as a ligand, bonding to metal ions. The resonance structures show how SCN- can bind using either its sulfur or nitrogen atom, making it a versatile bonding partner. Ongoing research continues to explore the details of SCN- interactions, suggesting that our understanding is constantly evolving.
Exploring SCN- Structure and Properties
Let’s delve deeper into the structural details of the thiocyanate ion (SCN-). It’s a linear molecule, with sulfur, carbon, and nitrogen arranged in a straight line. Carbon sits in the middle, bridged to sulfur by a likely double bond and to nitrogen by a likely triple bond (although a double bond representation is also considered).
Both sulfur and nitrogen have lone pairs of electrons—two on sulfur and one on nitrogen. The negative charge is delocalized, shared between the sulfur and nitrogen atoms. This resonance contributes significantly to SCN-‘s stability.
| Atom | Bonds to Carbon | Lone Pairs | Formal Charge (Approximate) |
|---|---|---|---|
| Sulfur (S) | Double Bond | 2 | Partial Negative |
| Carbon (C) | Double Bond to S, Double/likely Triple Bond to N | 0 | 0 |
| Nitrogen (N) | Double/likely Triple Bond | 1 | Partial Negative |
The formal charge is a bookkeeping tool for electrons, and in reality, the negative charge is spread out. Some research even suggests the structure might be more complex than current models indicate.
The linear shape (180-degree bond angle) results from the carbon atom’s sp hybridization. This structure is fundamental to SCN-‘s chemical behavior and interactions.
Unveiling the Electron Geometry of SCN-
The electron clouds around the central carbon in SCN- arrange themselves linearly, resulting in a linear electron geometry. This arrangement, combined with the absence of lone pairs on carbon, dictates the linear molecular shape of SCN-.
The sp hybridization of the central carbon atom plays a crucial role in this linear geometry. The hybrid orbitals form sigma bonds with sulfur and nitrogen, holding the ion together.
| Feature | Description |
|---|---|
| Electron Geometry | Linear |
| Molecular Shape | Linear |
| Hybridization | sp |
| Central Atom | Carbon |
This linear structure influences SCN-‘s chemical personality, allowing it to act as a good ligand, fitting into specific binding sites in larger molecules. The delocalization of charge through resonance further contributes to SCN-‘s stability, influencing its reactivity. Ongoing research is still exploring the finer points of SCN- behavior.
Does SCN- Follow the Octet Rule?
The octet rule suggests atoms prefer eight electrons in their outer shell. In SCN-, carbon and nitrogen generally adhere to this rule within the various resonance structures. However, sulfur sometimes has an “expanded octet,” accommodating more than eight electrons due to its access to d-orbitals.
Resonance structures provide a more complete picture of electron distribution. Formal charge analysis helps determine the most likely structures, with the negative charge preferably residing on the more electronegative nitrogen.
| Atom in SCN- | Follows Octet Rule? | Explanation |
|---|---|---|
| Carbon | Usually | Typically adheres to the octet rule in resonance structures. |
| Nitrogen | Usually | Typically adheres to the octet rule in resonance structures. |
| Sulfur | Sometimes | Can have an expanded octet due to access to d-orbitals. |
Sulfur’s expanded octet and the resonance structures influence SCN-‘s stability and behavior. The delocalized charge, facilitated by the expanded octet, contributes to the overall stability of the ion. While the octet rule is a good guideline, SCN- demonstrates that exceptions exist and can play a significant role in molecular stability. Ongoing research continues to refine our understanding of these concepts.
Want to test your chemistry knowledge? Try our stoichiometry worksheet and see how well you can apply your understanding of chemical reactions. Also, you can sort out the nucleotide building blocks by their name or classification by clicking on this link.
- Georgia Platform: A Southern Strategy, 1850s - March 31, 2025
- How many weeks is 40 days: Quick Conversion Guide for Accurate Results - March 31, 2025
- How many feet is 300 meters? 984 Feet: Understand Length Conversions Easily - March 31, 2025