This guide provides a comprehensive overview of the HNO Lewis structure, its geometry, bonding, and significance. We’ll explore the molecule’s architecture, unraveling its chemical behavior and interactions.
Decoding the HNO Lewis Structure
Let’s explore the Lewis structure of HNO, or nitroxyl. This structure serves as a blueprint, illustrating how atoms connect and providing clues to HNO’s chemical behavior.
Building the HNO Lewis Structure Step-by-Step
Constructing a Lewis structure is like assembling a puzzle.
1. Identifying the Central Atom: Nitrogen (N) is the central atom in HNO because it’s less electronegative than oxygen (O), meaning it’s more likely to share electrons.
2. Counting Valence Electrons: Valence electrons participate in bonding. Nitrogen has 5, oxygen has 6, and hydrogen has 1, totaling 12 valence electrons.
3. Forming Single Bonds: Draw single bonds between N-H and N-O, using 4 valence electrons.
4. Satisfying the Octet Rule: Atoms generally prefer 8 electrons in their outer shell (octet rule). Add three lone pairs to oxygen and one to nitrogen.
5. Creating a Double Bond: To satisfy nitrogen’s octet, convert one oxygen lone pair into a bonding pair, forming an N=O double bond.
The Final HNO Lewis Structure
The completed structure is H-N=O, with one lone pair on nitrogen and two on oxygen.
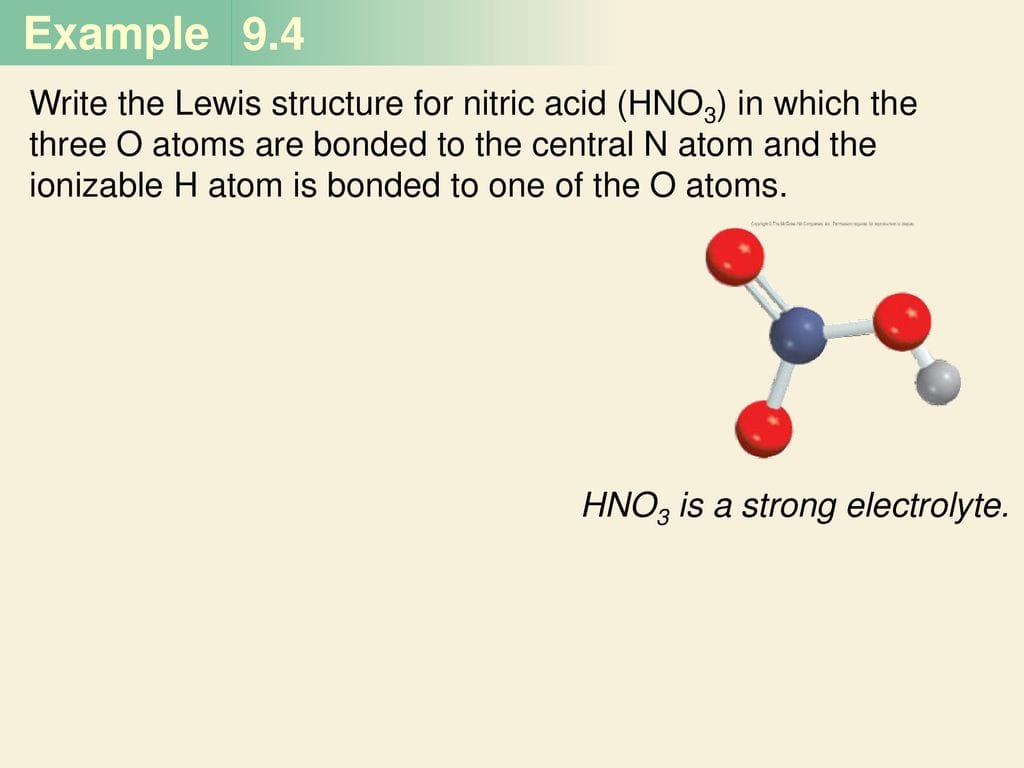
HNO vs. HNO3: Key Differences
HNO (nitroxyl) differs significantly from HNO3 (nitric acid). HNO3 has nitrogen bonded to three oxygens. This structural variation leads to distinct chemical properties. Think of it like building different structures with LEGOs – even with similar blocks, the results can vary dramatically.
Advanced Concepts: Hybridization and Ongoing Research
Nitrogen in HNO likely exhibits sp2 hybridization, suggesting a bond angle of approximately 120 degrees between H-N-O. However, the lone pair on nitrogen might slightly reduce this angle. Ongoing research continues to explore HNO’s behavior, and current theories are subject to further refinement. Unveiling the mysteries of chemical compounds? Visit our in-depth guide on hydrogen cyanide lewis structure to delve into its molecular makeup and unravel the intricacies of its bonding.
HNO Lewis Structure: A Simplified Explanation
Let’s break down the HNO Lewis structure construction:
Hydrogen (H), nitrogen (N), and oxygen (O) contribute 1, 5, and 6 valence electrons, respectively, totaling 12. Nitrogen is the central atom.
1. Connecting the Atoms: Create single bonds (N-H and N-O) using 4 electrons.
2. Completing Oxygen’s Octet: Add three lone pairs to oxygen, using the remaining 8 electrons.
3. Satisfying Nitrogen’s Octet: Convert one oxygen lone pair into a bonding pair, forming an N=O double bond.
The resulting structure is H-N=O, with nitrogen having one lone pair and oxygen having two. This structure suggests that HNO may exhibit both oxidizing and reducing properties.
HNO Geometry: Unveiling the Shape of Nitroxyl
HNO’s shape is bent, similar to a boomerang. This results from the lone pair on nitrogen repelling the bonded pairs, pushing the hydrogen and oxygen atoms closer.
Why HNO is Bent: The Lone Pair’s Influence
The lone pair on nitrogen exerts repulsive force on the bonding pairs, leading to the bent shape.
Hybridization and Bond Angles: A Deeper Look
Nitrogen in HNO undergoes sp2 hybridization, ideally leading to a trigonal planar arrangement. However, the lone pair causes the bent shape, with a bond angle of approximately 105 degrees. This bent geometry significantly impacts HNO’s polarity and interactions with other molecules.
Visualizing the Structure
- Draw the Lewis structure (H-N=O).
- Identify nitrogen’s sp2 hybridization.
- Recognize the bent geometry due to the lone pair.
- Predict a bond angle of around 105 degrees. There is debate among experts on the exact bond angle due to uncertainties associated with electron repulsion and the double bond.
The bent shape influences HNO’s chemical and biological behavior, affecting its polarity and dipole moment. Ongoing research continues to investigate HNO’s reactivity and potential therapeutic applications. Some experts believe that HNO’s unique bent structure may play a key role in its reactivity. It’s crucial to remember that scientific understanding is constantly evolving. What is the geometry of HNO? – hno lewis structure.
HNO Bonds Explained: A Simple Guide
HNO has three bonds: one N=O double bond and one N-H single bond.
Understanding HNO’s Structure
Nitrogen is the central atom, connected to hydrogen and oxygen. Twelve valence electrons participate in bonding, following the octet rule (mostly). Lone pairs on nitrogen (1) and oxygen (2) influence the molecule’s shape and reactivity.
Lewis Structure Construction
- Count total valence electrons (12).
- Place nitrogen (least electronegative) in the center.
- Connect atoms with single bonds.
- Distribute remaining electrons to satisfy octets (duet for hydrogen).
- Form a double bond (N=O) to complete nitrogen’s octet.
Molecular Geometry and Hybridization
The lone pair on nitrogen leads to a bent geometry, influencing the molecule’s properties. Nitrogen’s sp2 hybridization facilitates the double and single bonds. The H-N-O bond angle is probably around 109.5 degrees, slightly smaller due to lone pair repulsion.
Formal Charges
Formal charges on all atoms in HNO are zero, indicating a stable electron distribution. The reactivity of HNO may be influenced by the surrounding environment. How many bonds does HNO have? – hno lewis structure. Researchers are exploring HNO’s role in various biological processes, like vasodilation and neurotransmission. It’s important to note that scientific understanding is continuously evolving. Ongoing research continues to refine our understanding of this molecule, as the exact bond angle or details of its reactivity are still under investigation.
- Unlock Elemental 2 Secrets: Actionable Insights Now - April 2, 2025
- Lot’s Wife’s Name: Unveiling the Mystery of Sodom’s Fall - April 2, 2025
- Photocell Sensors: A Complete Guide for Selection and Implementation - April 2, 2025















1 thought on “Understanding the HNO Lewis Structure: A Complete Guide with Examples”
Comments are closed.