Instantly solve Boyle’s Law problems! Use our guide to understand the core principles and real-world implications of this essential gas law. We’ll cover everything from the basic formula to practical examples, helping you master gas calculations with ease.
Unlocking Boyle’s Law: Pressure & Volume’s Dance
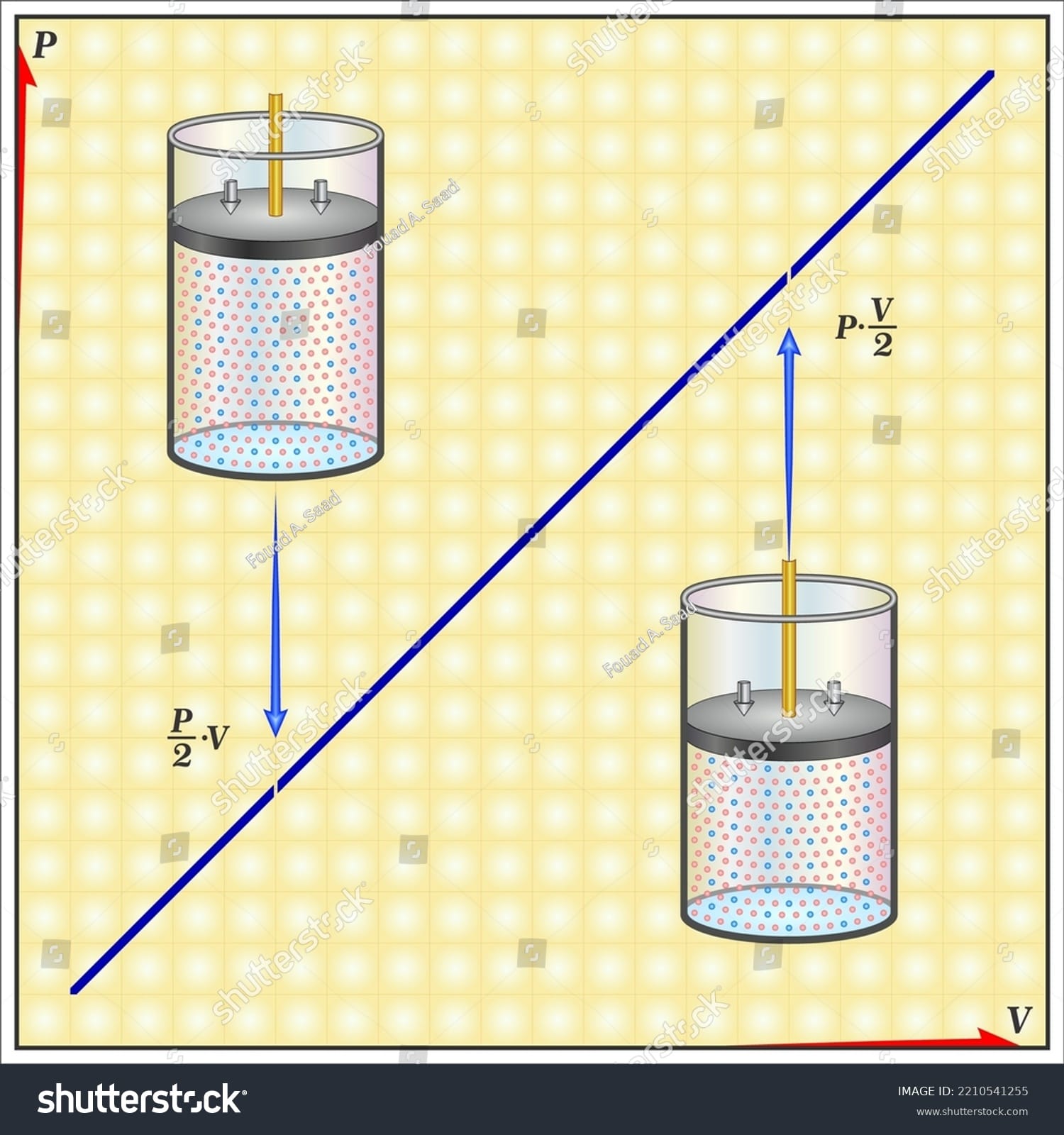
Think of a balloon. Squeeze it, and it gets smaller, right? That’s Boyle’s Law in action! It describes how the pressure and volume of a gas are linked when the temperature stays the same. More squeeze (higher pressure), less space (lower volume). This inverse relationship is fundamental to understanding how gases behave. Want to predict exactly how much a gas will shrink or expand? That’s where a Boyle’s Law calculator comes in handy.
Using a Boyle’s Law Calculator: A Step-by-Step Guide
A Boyle’s Law calculator is like having a physics wizard in your pocket. You plug in what you know, and it reveals the secrets of pressure and volume changes. Here’s the spell:
Know Your Variables: You’ll need three out of these four: initial pressure (P₁), initial volume (V₁), final pressure (P₂), and final volume (V₂). Make sure your units match—the calculator might prefer liters for volume and atmospheres for pressure, for instance.
Enter the Data: Input your known values into the calculator. Most calculators have clear labels to guide you.
Choose your Units: Select the units you want for the answer. Liters, milliliters, atmospheres, kilopascals—the options are usually there.
Calculate the Magic: Click “Calculate,” and poof – the answer appears! The calculator uses the formula P₁V₁ = P₂V₂ to work its magic.
Boyle’s Law in Action: Real-World Wonders
Boyle’s Law isn’t just theoretical; it’s all around us!
Scuba Diving: Deeper dives mean higher water pressure. This compresses the air in a diver’s lungs (reducing volume), highlighting why careful ascent is crucial to avoid decompression sickness. This relationship between depth, pressure, and lung volume is a critical application of Boyle’s law.
Syringes: Pulling the plunger creates lower pressure inside the syringe. This allows the volume inside to increase, drawing fluid in, perfectly illustrating Boyle’s Law.
Weather Balloons: These balloons expand as they ascend because atmospheric pressure decreases with altitude. Lower pressure outside allows the gas inside the balloon to expand, increasing its volume and allowing it to float high above.
Aerosol Cans: The contents are under high pressure. Pressing the button releases this pressure, allowing the product inside to expand rapidly and propel outwards. This dramatic change in volume at a lower pressure shows Boyle’s Law at work.
Boyle’s Law: The Fine Print
Boyle’s Law works best for “ideal” gases – theoretical gases that perfectly follow all gas laws. Real gases, especially at high pressures or low temperatures, may deviate slightly. Also, remember temperature must stay constant for Boyle’s Law to apply. If the temperature changes, so does the relationship between pressure and volume. Current research suggests that intermolecular forces and the size of gas molecules likely contribute to these deviations.
Calculating Boyle’s Law: The Formula Unveiled
Boyle’s Law says that at a constant temperature, squeezing a gas (decreasing volume) increases its pressure. The relationship is described by the equation:
P₁V₁ = P₂V₂
- P₁: Initial pressure
- V₁: Initial volume
- P₂: Final pressure
- V₂: Final volume
This equation represents the balance between the initial and final states of the gas. To find any one of these variables, simply rearrange the equation:
- Finding P₂: P₂ = (P₁V₁) / V₂
- Finding V₂: V₂ = (P₁V₁) / P₂
- Finding P₁: P₁ = (P₂V₂) / V₁
- Finding V₁: V₁ = (P₂V₂) / P₁
Finding P₂: A Worked Example
Let’s say you have a balloon with an initial pressure of 1 atmosphere (P₁) and a volume of 1 liter (V₁). You squeeze it down to 0.5 liters (V₂). What’s the new pressure (P₂)?
P₂ = (1 atm * 1 L) / 0.5 L = 2 atm. Squeezing the balloon doubled the pressure!
| Factor | Description |
|---|---|
| Initial Pressure (P₁) | The pressure of the gas before any changes are made. |
| Initial Volume (V₁) | The volume of the gas before any changes are made. |
| Final Pressure (P₂) | The pressure of the gas after a change in volume. |
| Final Volume (V₂) | The volume of the gas after a change in pressure. |
| Constant Temperature (T) | It’s crucial that the temperature of the gas remains the same throughout the entire process. |
Understanding the Boyle’s Law Formula: P₁V₁ = P₂V₂
This simple but powerful formula is the key to unlocking Boyle’s Law:
- P₁: Initial pressure (before any change)
- V₁: Initial volume (before any change)
- P₂: Final pressure (after the change)
- V₂: Final volume (after the change)
The formula states that the product of the initial pressure and volume equals the product of the final pressure and volume, as long as the temperature remains constant. This relationship highlights the inverse proportionality between pressure and volume.
Here’s a table summarizing the key elements:
| Variable | Description |
|---|---|
| P₁ | Initial Pressure |
| V₁ | Initial Volume |
| P₂ | Final Pressure |
| V₂ | Final Volume |
We are sure that you would also want to check our CEFM Exam prep study guide for more information.
- Georgia Platform: A Southern Strategy, 1850s - March 31, 2025
- How many weeks is 40 days: Quick Conversion Guide for Accurate Results - March 31, 2025
- How many feet is 300 meters? 984 Feet: Understand Length Conversions Easily - March 31, 2025