Unpacking BI3: A Closer Look at Boron Triiodide
Boron triiodide (BI3) is a chemical compound with the formula BI3. It exists as a colorless, crystalline solid at room temperature and holds significant importance in organic synthesis and materials science. [https://www.lolaapp.com/organic-synthesis/]. This article delves into the properties, applications, and intriguing chemistry of this versatile compound.
BI3: Structure, Reactivity, and Applications
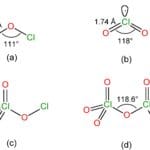
The molecular geometry of BI3 is trigonal planar, which means that the boron atom is positioned at the center and surrounded by three iodine atoms in a flat, triangular arrangement. This unique structure influences the compound’s reactivity. BI3 is categorized as a strong Lewis acid, indicating its ability to accept electron pairs from Lewis bases. This characteristic stems from the electron deficiency of the boron atom, which possesses an incomplete octet of electrons. As such, it readily seeks to attract electron pairs from other molecules.
BI3 as a Catalyst and Precursor
The strong Lewis acidity of BI3 makes it an effective catalyst in a variety of organic reactions. For instance, it can catalyze the cleavage of ethers, a process where the oxygen atom in an ether molecule (R-O-R’) is broken, forming new bonds with other atoms or molecules. BI3’s electron-accepting nature allows it to facilitate the breaking and forming of chemical bonds, thereby accelerating the reaction rate.
In addition to its catalytic properties, BI3 serves as an important precursor for the synthesis of various boron-containing compounds. These compounds find applications in diverse fields, including pharmaceuticals, polymers, and materials science.
BI3 in Material Science
The importance of BI3 extends beyond the realm of organic chemistry. It plays a crucial role in materials science, particularly in the production of semiconductors and other electronic materials. The precise mechanisms by which BI3 contributes to these processes can be complex, but its unique chemical properties make it an essential component in the manufacturing of many high-tech devices [https://www.lolaapp.com/semiconductors/].
Nomenclature and Identification
- Chemical Formula: BI3
- IUPAC Name: Boron Triiodide
- Other Names: Triiodoborane, Borane, triiodo-
- CAS Registry Number: 13517-10-7
Physical and Chemical Properties
- Molecular Weight: 391.52441 g/mol
- Appearance: Colorless, crystalline solid
- Melting Point: 49.9 °C (121.8 °F)
- Boiling Point: 210 °C (410 °F)
- Solubility: Soluble in carbon disulfide and nonpolar solvents
- Chemical Properties: Strong Lewis acid (electron acceptor), reducing agent
Preparation
BI3 can be synthesized through several methods:
- Direct reaction of boron with iodine at elevated temperatures (approximately 209.5 °C or 409.1 °F).
- Reaction of hydroiodic acid (HI) with boron trichloride (BCl3).
Safety Precautions
Given BI3’s reactivity, it’s essential to handle it with care. The compound is corrosive and can react with certain materials, including human tissue. Therefore, appropriate safety measures should be in place when working with it. Always wear gloves and eye protection, and ensure proper ventilation in the working area.
Comparing BI3 to Other Boron Halides
BI3 is a member of the boron halide family, which includes boron trifluoride (BF3) and boron trichloride (BCl3). While all three compounds share a similar trigonal planar geometry, they exhibit differences in Lewis acidity and reactivity. This variation can be attributed to the size and electronegativity of the halogen atoms (fluorine, chlorine, and iodine).
The following table summarizes the key differences:
| Boron Halide | Lewis Acidity | Reactivity | Stability |
|---|---|---|---|
| BF3 | Moderate | High | High |
| BCl3 | Intermediate | Moderate | Moderate |
| BI3 | Strong | High | Low |
The larger size and lower electronegativity of iodine compared to fluorine and chlorine contribute to the stronger Lewis acidity of BI3. The iodine atoms hold onto their electrons less tightly, allowing the boron atom to more readily accept electron pairs from other molecules.
Ongoing Research and Future Directions
Despite our current understanding of BI3, research on this intriguing compound is ongoing. Scientists are actively investigating its catalytic mechanisms in various organic reactions, exploring its potential in developing novel materials, and studying its interactions with other chemical species.
Conclusion
Boron triiodide (BI3) is a chemically intriguing and versatile compound. Its unique properties, including its strong Lewis acidity and reactivity, make it a valuable tool in organic synthesis, materials science, and other fields. As research continues to uncover the full scope of BI3’s capabilities, we can anticipate further exciting discoveries and applications for this remarkable compound.
- Unlock Elemental 2 Secrets: Actionable Insights Now - April 2, 2025
- Lot’s Wife’s Name: Unveiling the Mystery of Sodom’s Fall - April 2, 2025
- Photocell Sensors: A Complete Guide for Selection and Implementation - April 2, 2025