Decoding Argon’s Stability: The Role of its Lewis Dot Structure
Everything around us, from the air we breathe to the objects we touch, is composed of atoms and molecules. These tiny building blocks interact in fascinating ways, and understanding their behavior is key to unlocking the secrets of the universe. In this guide, we’ll delve into the world of argon, a noble gas, and explore its Lewis dot structure—a visual representation that reveals the secrets of its stability and inertness.
Why is Argon So Unreactive?
Argon stands out in the periodic table as a loner, an element that rarely interacts with others. This aloofness is due to its electron configuration and, more specifically, its Lewis dot structure. The Lewis dot structure provides a simplified yet powerful way to visualize the valence electrons—the electrons in the outermost shell—which are responsible for chemical bonding. Think of these valence electrons as the atom’s social tentacles, reaching out to connect with other atoms.
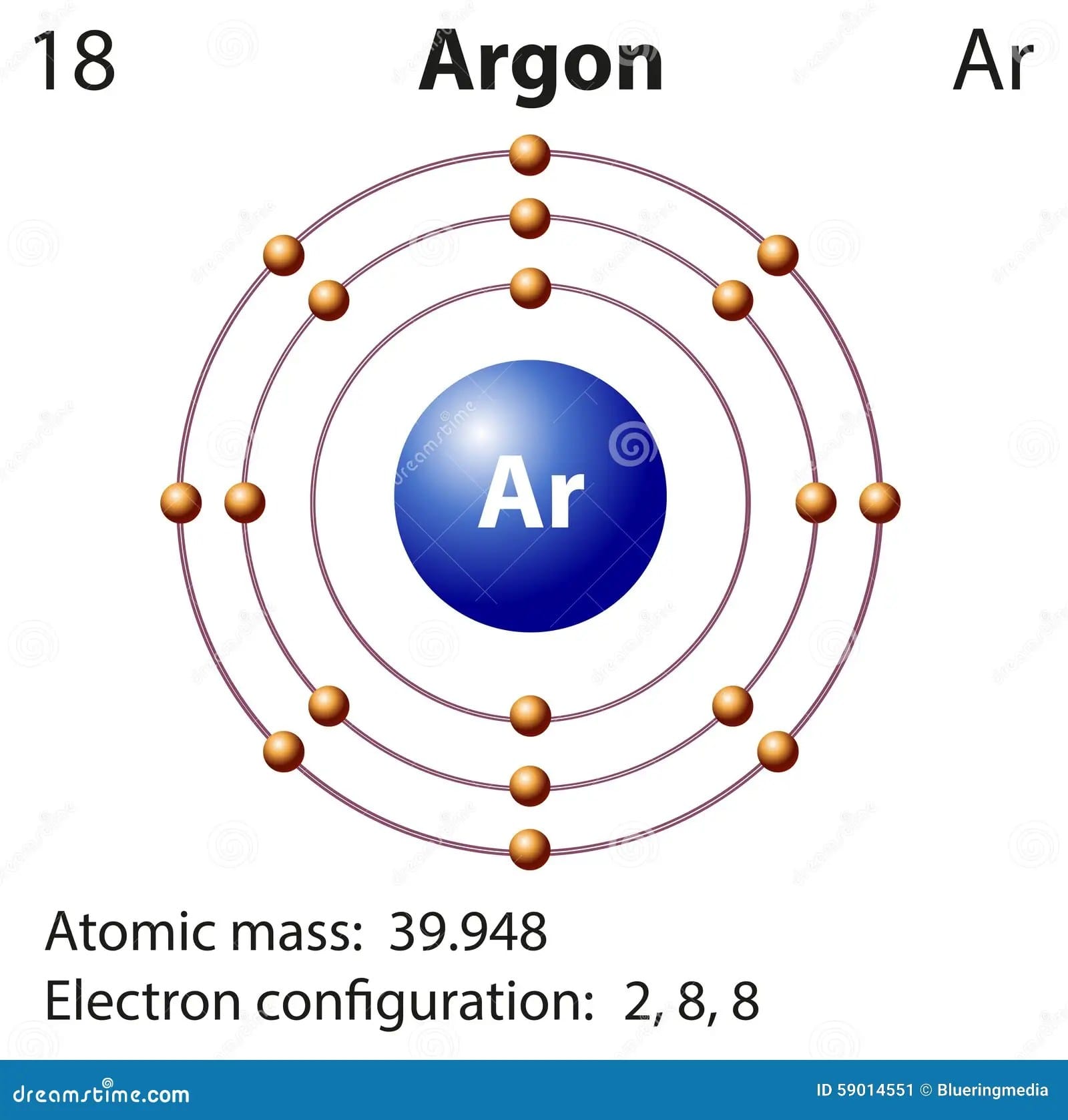
Argon’s Lewis dot structure is remarkably simple: the symbol “Ar” surrounded by eight dots. These eight dots represent argon’s eight valence electrons, a “full house” in the atomic world. This complete octet signifies a state of exceptional stability. Just like a puzzle perfectly completed, argon has no need to gain, lose, or share electrons with other atoms. This is why argon is considered chemically inert.
This inherent stability makes argon incredibly useful. It’s used in welding to create a protective, non-reactive atmosphere, preventing the hot metal from oxidizing. Inside incandescent light bulbs, argon prolongs the filament’s life by preventing it from burning away. Even in museums, argon helps protect precious artifacts from decay by displacing reactive oxygen and moisture.
Drawing Argon’s Lewis Dot Structure: A Step-by-Step Guide
So, how do we construct argon’s Lewis dot structure? It’s easier than you might think!
Start with the symbol: Write down “Ar” to represent Argon.
Add the dots: Argon has 18 electrons total. Only eight of these are in the outermost shell (the valence electrons). These are the ones involved in chemical bonding (or the lack thereof!). Place eight dots around the “Ar”, distributing them symmetrically—one on each side and one on each corner, like a box with a dot on each side and one on each corner. This visually represents the complete octet.
This simple diagram helps us grasp why argon is so unreactive. The full octet of electrons suggests that argon is content with its current electron configuration and has no desire to interact with other atoms.
Argon’s 8 Dots: The Key to Inertness
Let’s focus on those eight dots surrounding “Ar”. Why are they so important? Those eight dots represent the eight valence electrons in argon’s outermost electron shell. This full complement of valence electrons is what gives argon its remarkable stability and inertness.
Atoms generally strive to achieve a full outer shell, often containing eight electrons (the “octet rule”). This is because a full outer shell represents a low-energy, stable state. Argon, unlike many other elements, already possesses this stable configuration. This means it has no need to participate in chemical reactions to gain, lose, or share electrons.
This inherent stability is the hallmark of the noble gases, the exclusive group to which argon belongs (Group 18 in the periodic table). All noble gases share this characteristic of having a full outermost electron shell, which explains their low reactivity. While the octet rule is a useful guideline, there are exceptions, especially with elements beyond the second row of the periodic table. Some elements can exhibit “expanded octets,” accommodating more than eight valence electrons. Ongoing research continues to explore the complexities of chemical bonding.
Argon’s full octet, visualized by the eight dots in its Lewis dot structure, is the key to understanding its inert nature and its diverse applications, from welding and lighting to preserving historical documents. Prepare for mind-blowing facts about the chemical composition of the bi3 compound name and its unique properties that will leave you in awe.
Argon’s Electron Configuration and Its Implications
Defining Electron Configuration
Electron configuration provides a roadmap of how electrons are arranged within an atom’s various energy levels and subshells. It uses a specific notation (e.g., 1s²2s²2p⁶3s²3p⁶ for Argon), where the numbers represent the principal quantum number (energy level), the letters (s, p, d, f) represent subshells, and the superscripts indicate the number of electrons in each subshell.
Argon’s Configuration: The Basis of Inertness
Argon, with its 18 electrons, has the electron configuration 1s²2s²2p⁶3s²3p⁶. The shorthand notation, [Ne]3s²3p⁶, uses the preceding noble gas, Neon ([Ne]), as a shortcut. The crucial takeaway is the filled outer shell: 3s²3p⁶ adds up to eight electrons in the outermost (3rd) energy level—these are argon’s valence electrons. This full octet is the reason why argon is so stable and reluctant to interact with other elements.
This electronic arrangement explains why argon, and other noble gases, tend to exist in their pure, uncombined forms in nature. Their inherent stability makes them reluctant participants in chemical reactions.
Connecting Configuration and the Lewis Structure
The Lewis dot structure, with its eight dots around “Ar”, provides a visual representation of that crucial 3s²3p⁶ configuration. It simplifies a complex quantum mechanical model into an easily understandable diagram.
While simple Lewis structures are useful, they have limitations, particularly when dealing with heavier elements or more complex molecules. Ongoing research continues to refine our understanding of electron behavior, but the basic principles illustrated by the Lewis structure remain powerful tools for predicting and explaining chemical reactivity.
Key Points:
- Argon, a noble gas, is chemically inert due to its full outer electron shell.
- Its Lewis dot structure features eight dots around the symbol “Ar,” representing the eight valence electrons.
- This full octet of valence electrons makes argon incredibly stable and unreactive.
- Argon’s inertness finds practical applications in welding, incandescent light bulbs, and preserving artifacts.
- The Lewis dot structure simplifies the complex concept of electron configuration, making it easier to understand argon’s chemical behavior.
This expanded and restructured guide provides a more comprehensive understanding of argon’s Lewis dot structure and its connection to argon’s stability and real-world applications. The inclusion of electron configurations, step-by-step instructions, and clear explanations enhances the learning experience. Remember, while models like the Lewis dot structure are valuable tools, they represent a simplified view of a complex reality. Current research continues to deepen our understanding of chemical bonding, and our knowledge may continue to evolve.
- Senior at What Age: Benefits & Eligibility Guide - March 29, 2025
- Unlocking Senior Benefits: How Old is a Senior? Your Complete Guide - March 29, 2025
- Master Russian Politeness:A Guide to Saying Please - March 29, 2025